Bridging the Age Gap in Breast Cancer
Bridging the Age Gap is an NIHR funded study that aims to optimize the management of older women and reduce the age-gap in cancer outcomes between older and younger women with breast cancer.
We are doing this by:
- Developing a predictive tool to tailor treatment options for older women according to breast cancer factors and their fitness/frailty
- Developing a Decision Support Instrument (DESI) to assist older women making informed decisions about their preferred treatment
The study was open in 57 centres around England and Wales, and finished recruiting in June 2018. Analysis is now underway and our key papers and results will be made available later this year.
Phase 2
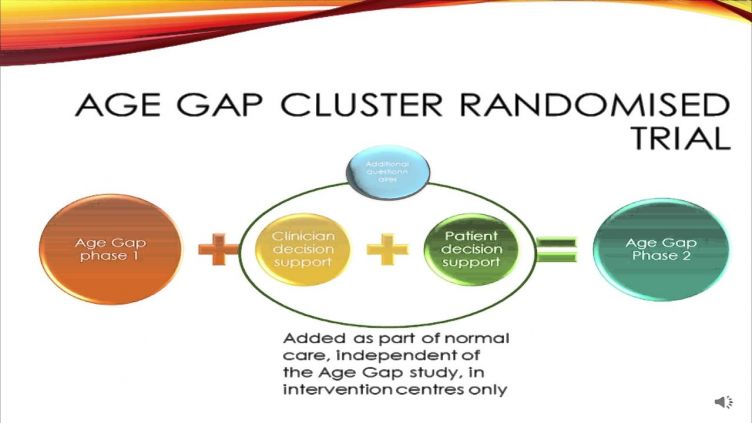
The Bridging the Age Gap in Breast Cancer study is a multicentre cohort study recruiting up to 3000 older women (>70) with operable breast cancer at 50 sites in the UK. Its aim is to collect detailed data about the personal and cancer characteristics of these women, the treatment they receive and what their short, medium and long term outcomes are. The data will be used to help to optimise the care of these older women so treatment can be better tailored to their health and fitness levels, reducing some of the wide practice variation that currently exists across the UK.
The second phase, which will be embedded within the existing study, will be to evaluate whether use of a package of decision support interventions (DESIs), given to 50% of existing sites and embedded as ‘standard of care’, helps to improve the quality of life, decision quality, decision regret, satisfaction and treatment understanding of older women entering the Age Gap study. These DESIs will be aimed at women facing a choice of surgery or primary endocrine therapy (PET) or, following surgery, for those with higher risk cancer facing a choice of chemotherapy or no chemotherapy. These are the two areas where clinical practice in older women differs most markedly from that in younger women and where there are high levels of variation between breast units.
A package of decision support interventions (DESIs) have been developed and will form a resource to be implemented in half of the Age Gap recruiting sites as part of standard care. These sites will be trained in their use which will become a routine part of the counselling they are able to offer all women, whether they are in the Age Gap study or not.
The DESIs comprise 2 patient facing booklets designed especially for older women facing these choices and 2 web based algorithms (a bit like PREDICT or adjuvant on line and just as easy to use) which may be used by the clinical team to predict individual risk and benefit information that may be shared with an individual patient. These resources have been carefully developed using the best available evidence and have undergone extensive user testing. Sites will be allocated at random to have access to use these decision tools as part of routine care or simply continue with normal best practice pre-treatment counselling.
Recruitment, eligibility and data collection for the study will largely be unchanged with all of the current outcomes collected. The only changes are the addition of a few new questionnaires about their pre treatment counselling and decision making and the ability to use the decision aids if they wish to help patients decide on treatment (optional and only if the patient is facing that particular choice) in half of centres. staff and patients in a small percentage of selected sites will be asked to give feedback about the resources as part of a formal process evaluation.
We expect that recruitment to the main study will have reached 2000 by the time we introduce the DESIs into 50% of our sites in October 2015 (based on a recruitment rate of about 70 per month and the fact that we are just over 1700 now). The study will continue to run for a further 24 months and recruit a further 1600 cases. Most of the data from these phase 2 patients will be co-analysed with the main cohort, but sub-group analysis will take place to compare outcomes in the intervention sites and non intervention sites. We expect recruitment rates to be similar in phases 1 and 2 as the study is largely unchanged other than that staff will have access to the DESIs to use alongside their routine counselling resources as part of normal practice.
Phase 2 Training
We have now received ethics approval for Phase 2 of the Age Gap study. The cohort study and its eligibility criteria remain the same but half of all sites will be offered access to several tools to help older women make treatment choices.
In October 2015 we invited members of local teams to attend one of 3 training /trial update sessions held in London, Manchester and Sheffield. Since then, the team have been on an intensive round of visits to initiate sites to the new protocol.
For site staff who were unable to attend one of our visits, there are resources available on the web tool website which can be watched for an update on the new protocol. We are also happy to come out to sites to explain the new protocol if this would be helpful. Please make contact the with main trial team to make arrangements.
We have been extremely pleased with the level of enthusiasm that sites have shown for the new study and the feedback for the decision tools has been very positive. We are especially keen to engage with the clinical nurse specialists in recruiting centres and also oncologists, as the latter in particular are likely to be involved in helping with chemotherapy decision support which is an integral part of the study.
- GDPR
-
Information about Data Usage
Study Title: Bridging the Age Gap in Breast Cancer: Improving outcomes for older women
IRAS: 115550Doncaster and Bassetlaw Teaching Hospitals NHS Foundation Trust (DBTH) is the Sponsor for this study based in the United Kingdom. We will be using information from you and your medical records in order to undertake this study and will act as the data controller for this study. The data management is delegated to the Clinical Trials Research Unit (CTRU) in the School of Health and Related Research at The University of Sheffield. Together we are responsible for looking after your information and using it properly. Identifiable information about you will be kept for 5 years after the study has finished.
Your rights to access, change or move your information are limited, as we need to manage your information in specific ways in order for the research to be reliable and accurate. If you withdraw from the study, we will keep the information about you that we have already obtained. To safeguard your rights, we will use the minimum personally-identifiable information possible.
You can find out more about how we use your information by following the links below:
https://www.dbth.nhs.uk/about-us/our-publications/uk-data-protection-legislation-eu-general-data-protection-regulation-gdpr/
https://www.sheffield.ac.uk/govern/data-protection/privacy/generalThe recruiting centre will collect information from you and your medical records including your name, NHS number and contact details, for this research study in accordance with our instructions. The recruiting centre will use this information as needed, to contact you about the research study, and make sure that relevant information about the study is recorded for your care, and to oversee the quality of the study. Certain individuals from DBTH, the University of Sheffield (on behalf of DBTH as Sponsor) and regulatory organisations may look at your medical and research records to check the accuracy of the research study.
The recruiting centre will pass the information collected from you and your medical records to the University of Sheffield. The University of Sheffield will also be requesting follow-up data about mortality, causes of death and hospital episode statistics from NHS Digital, and will act as the data controller for this information. Data will be processed in accordance with the University privacy notice. The only people in the University of Sheffield who will have access to information that identifies you will be people responsible for auditing the data collection process. The people who analyse the information will not be able to identify you and will not be able to find out your name, or contact details.
The recruiting centre will keep identifiable information about you from this study 5 years after the study has finished.
*The recruiting centre refers to the NHS Trust/hospital where the participant was recruited.
Recent publications
- Quality of life versus length of life considerations in cancer patients: A systematic literature review. Shrestha A, Martin C, Burton M, Walters S, Collins K, Wyld L. Psychooncology. 2019 Mar 5.
- How are caregivers involved in treatment decision making for older people with dementia and a new diagnosis of cancer? Martin C, Shrestha A, Burton M, Collins K, Wyld L. Psychooncology. 2019 Jun;28(6):1197-1206.
- Adjuvant Chemotherapy for Breast Cancer in Older Women: An Analysis of Retrospective English Cancer Registration Data. Ward SE, Holmes GR, Ring A, Richards PD, Morgan JL, Broggio JW, Collins K, Reed MWR, Wyld L. Clin Oncol (R Coll Radiol). 2019 Jul;31(7):444-452.
- Kate J Lifford, Adrian Edwards, Maria Burton, Helena Harder, Fiona Armitage, Jenna L Morgan, Lisa Caldon , Kirsty Balachandran Alistair Ring Karen Collins Malcolm Reed Lynda Wyld, Kate Brain. Efficient development and usability testing of decision support interventions for older women with breast cancer. Patient Preference and Adherence 2019:13 131–143
- Omission of surgery in older women with early breast cancer has an adverse impact on breast cancer-specific survival. Ward SE, Richards PD, Morgan JL, Holmes GR, Broggio JW, Collins K, Reed MWR, Wyld L. Br J Surg. 2018 Oct;105(11):1454-1463
- Collins K, Reed M, Lifford K, Burton M, Edwards A, Ring A, Brain K, Harder H, Robinson T, Cheung KL, Morgan J,
Audisio R, Ward S, Richards P, Martin C, Chater T, Pemberton K, Nettleship A, Murray C, Walters S, Bortolami O,
Armitage F, Leonard R, Gath J, Revell D, Green T, Wyld L. Bridging the age gap in breast cancer: evaluation of decision support interventions for older women with operable breast cancer: protocol for a cluster randomised controlled trial. BMJ Open. 2017 Jul 31;7(7). - Morgan JL, Walters SJ, Collins K, Robinson TG, Cheung KL, Audisio R, Reed MW, Wyld L. What influences healthcare professionals' treatment preferences for older women with operable breast cancer? An application of the discrete choice experiment. Eur J Surg Oncol. 2017 Jul;43(7):1282-1287.
- Burton M, Kilner K, Wyld L, Lifford KJ, Gordon F, Allison A, Reed M, Collins KA. Information needs and decision-making preferences of older women offered a choice between surgery and primary endocrine therapy for early breast cancer. Psychooncology. 2017 Mar 23
- Richards P, Ward S, Morgan J, Lagord C, Reed M, Collins K, Wyld L. The use of surgery in the treatment of ER+ early stage breast cancer in England: Variation by time, age and patient characteristics. Eur J Surg Oncol. 2016
Apr;42(4):489-96 - Morgan JL, Richards P, Zaman O, Ward S, Collins K, Robinson T, Cheung KL, Audisio RA, Reed MW, Wyld L. The
decision-making process for senior cancer patients: treatment allocation of older women with operable breast cancer in the UK. Cancer Biol Med. 2015 Dec;12(4):308-15. - Morgan JL, Collins K, Robinson TG, Cheung KL, Audisio R, Reed MW, Wyld L. Healthcare professionals' preferences for surgery or primary endocrine therapy to treat older women with operable breast cancer. Eur J Surg Oncol. 2015
Sep;41(9):1234-42 - Morgan J, Richards P, Ward S, Francis M, Lawrence G, Collins K, Reed M, Wyld L. Case-mix analysis and variation in rates of non-surgical treatment of older women with operable breast cancer. Br J Surg. 2015 Aug;102(9):1056-63.
- Morgan JL, Burton M, Collins K, Lifford KJ, Robinson TG, Cheung KL, Audisio R, Reed MW, Wyld L. The balance of
clinician and patient input into treatment decision-making in older women with operable breast cancer. Psychooncology. 2015 - Lifford KJ, Witt J, Burton M, Collins K, Caldon L, Edwards A, Reed M, Wyld L, Brain K. Understanding older women's
decision making and coping in the context of breast cancer treatment. BMC Med Inform Decis Mak. 2015 Jun
10;15:45. - Audisio RA, Wyld L. No standard is set for older women with breast cancer. Eur J Surg Oncol. 2015 May;41(5):607-9.
- Morgan, J, Wyld L, Reed MW. Surgery versus primary endocrine therapy for operable primary breast cancer in elderly women (70 years plus). Updated review, 2014. The Cochrane Database of Systematic Reviews.
- Morgan JL, Reed MW, Wyld L. Primary endocrine therapy as a treatment for older women with operable breast
cancer - a comparison of randomised controlled trial and cohort study findings. Eur J Surg Oncol. 2014, 40(6):676-84 - Burton M, Collins K, Caldon L, Wyld L, Reed M. Information Needs of Older Women Faced with a Choice of Primary
Endocrine Therapy or Surgery for Early-Stage Breast Cancer: A Lit. Review. Curr Breast Cancer Rep 2014:6:235–244. - Burton M, Collins KA, Lifford KJ, Brain K, Wyld L, Caldon L, Gath J, Revell D, Reed MW. The information and decision support needs of older women (>75 yrs) facing treatment choices for breast cancer: a qualitative study.
Psychooncology. 2015 Aug;24(8):878-84.
Study contacts
Study leads
- Prof. Lynda Wyld, l.wyld@sheffield.ac.uk, +44 114 2268640
- Prof. Malcolm Reed, m.reed@bsms.ac.uk
Trial manager
- Ms. Charlene Martin, c.l.martin@sheffield.ac.uk, +44 114 215 9013
Phase 2 Lead Researcher
Get in touch
Email: agegap@sheffield.ac.uk or Charlene Martin: +44 (0)114 215 9013