Intercalated BMedSci degree
An intercalated degree involves an extra year of study, which is inserted between the years of the Bachelor of Dental Surgery (BDS) course, leading to the qualification of BMedSci.

Intercalation is an opportunity to obtain a deeper understanding of one of the subjects introduced in the BDS dental course, and will allow you to undertake a substantial research project. Research forms an essential part of your dental training, and this degree presents an early opportunity to develop research skills and potential.
An intercalated degree will also considerably enhance your CV, and broaden employment prospects. If you're interested in pursuing an academic career, it is even more worthwhile.
Although intercalating students do very diverse projects, all students have common aims and objectives that are fostered in the mandatory short course and in the supervisory element of the programme, as well as the your own self-directed study.
BMedSci (intercalation) projects for 2024/25
- Development of an innervated 3D tissue-engineered oral mucosa model
-
Supervisors: Prof FM Boissonade, Prof DW Lambert, Prof C Murdoch and Dr HE Colley
Background
Over the last decade our understanding of epithelial and stromal cell interaction has been greatly enhanced by the development of a wide range of 3D tissue-engineered constructs including models of skin and oral mucosa. These 3D tissue-engineered models can be used for a wide variety of purposes such as studies of toxicity, wound healing, tumour biology, and other skin disease. In addition to their uses for in vitro investigations, they are also beginning to deliver benefit in the clinic (usually in small-scale reconstructive surgery procedures). A number of cell types have been incorporated into these systems, thus allowing a closer approximation to the conditions found in human skin and oral mucosa in vivo. These cell types include keratinocytes, dermal fibroblasts and a range of immune cells. However to date there are very few models of skin or oral mucosa that incorporate neuronal cells.
There is now growing evidence that interaction between keratinocytes, fibroblasts and sensory neurones plays a significant role in both skin homeostasis and disease (eg wound healing, eczema and psoriasis). The neuropeptides calcitonin gene-related peptide (CGRP) and substance P have an established role in neurogenic inflammation and are thought to influence proliferation and morphogenesis of both keratinocytes and fibroblasts. Is it also well known that skin and oral mucosal cells produce a wide range of neurotrophic factors that have significant influence on neuronal development.
Aims
To develop an innervated 3D tissue- engineered oral mucosa model, and study the effects of innervation on skin and mucosal morphology and response to injury.
Techniques
A variety of cell culture techniques will be used. 3D oral mucosa and trigeminal ganglion neurones will be cultured using techniques developed in our laboratories. Successful co culture will be determined by immunohistochemical identification of different cell types using methods that are well established in our laboratories.
References
- MacNeil S. Progress and opportunities for tissue-engineered skin. Nature 2007;445:874-880
- Roggenkamp D, Kopnick S, Stab F et al. Epidermal nerve fibres modulate keratinocyte growth via neuropeptide signaling in an innervated skin model.
J Investigative Dermatology 2013;133:1620-1628 - Truzzi F, Marconi A, Pincelli C. Neurotrophins in healthy and diseased skin. Dermato-Endocrinology 2011;3:32-36
- Yu XJ, Li CY, Xu YH, et al. Calcitonin gene-related peptide increases proliferation of human HaCaT keratinocytes by activation of MAP kinases.
Cell Biol Int. 2009;33:1144-8 - Colley HE, Eves PC, Pinnock A, Thornhill MH, Murdoch C. Tissue-engineered oral mucosa to study radiotherapy-induced oral mucositis. Int J Radiat Biol. 2013 Nov;89(11):907-14.
External Partner Details: Not applicable
Use of human tissue: Yes
HBV vaccination required: Yes
Ethics Approval: In place
For further information, contact Professor F Boissonade at f.boissonade@sheffield.ac.uk
- Neural–stromal interactions in tumour progression
-
Supervisors: Professor FM Boissonade and Professor DW Lambert
Background
There is growing evidence that neural mediators play a role in the tumour microenvironment, and they have been implicated in tumour progression in breast, pancreatic and prostate cancer. However, the molecular mechanisms underlying their effects are poorly understood. Our recent studies have demonstrated significant cross-talk between neurones and oral squamous cell carcinoma (OSCC) cells.
We have identified receptors for specific neural mediators in OSCC-derived cell lines and have demonstrated that neural mediators have significant chemoattractant and proliferative effects on these cell lines. We have also shown that nerve growth factor (NGF) is elevated in OSCC-derived cell lines (relative to normal oral keratinocytes), and that NGF secretion from cancer-associated fibroblasts (CAFs) is significantly increased compared with that from normal oral fibroblasts. These findings suggest that neural mediators are capable of enhancing the proliferation and migration of OSCC cells – potentially enhancing tumour progression – and that OSCC cells and CAFs may influence nerve growth in tumours. Thus neuronal factors may have potential as therapeutic targets for cancer treatment.
Aim
To further evaluate the effects of neural mediators and their antagonists on the proliferation and migration of OSCC-derived cell lines alone and in the presence of other cell types, and to identify the mechanisms underlying these effects.
Techniques
Quantitative polymerase chain reaction (qPCR) and immunocytochemistry will be used to identify specific neural mediators and components of their receptors in normal oral keratinocytes (NOKs); dysplastic, cancerous and metastatic cell lines; and neurones. Co-culture systems, cell proliferation and migration assays will be used to identify functional cross-talk between neurones, cancer cells and fibroblasts (the predominant cell type of the tumour microenvironment). Enzyme-linked immunosorbent assays (ELISA) and qPCR will be employed to identify secreted factors involved in neural–tumour interactions.
This project will identify specific neural mediators and receptors that affect OSCC cell migration and proliferation and that may provide novel targets for cancer treatment.
References
- Hoffmann P, Hoeck K, Deters S, et al. Substance P and calcitonin gene-related peptides induce TGF-alpha expression in epithelial cells via mast cells and fibroblasts. Regul Pept 2010; 161: 33–37.
- Magnon C, Hall SJ, Lin J, et al. Autonomic nerve development contributes to prostate cancer progression. Science 2013; 341: 1236361.
- Toda M, Suzuki T, Hosono K, et al. Neuronal system-dependent facilitation of tumour angiogenesis and tumour growth by calcitonin gene-related peptide. Proc Natl Acad Sci USA 2008; 105: 13550–13555.
- Venkatesh H, Monje M. Neuronal activity in ontogeny and oncology. Trends Cancer 2017; 3: 89–112.
- Yu XJ, Li CY, Xu YH, et al. Calcitonin gene-related peptide increases the proliferation of human HaCaT keratinocytes by activation of MAP kinases. Cell Biol Int 2009; 33: 1144–1148.
External Partner Details: Not applicable
Use of human tissue: Yes
HBV vaccination required: Yes
Ethics Approval: In place
For further information please contact Professor F Boissonade at f.boissonade@sheffield.ac.uk
-
Prognostic potential of R-loop expression in oral epithelial dysplasia and early invasive oral squamous cell carcinoma
-
Supervisors: Dr H Crane and Professor A Khurram
Background
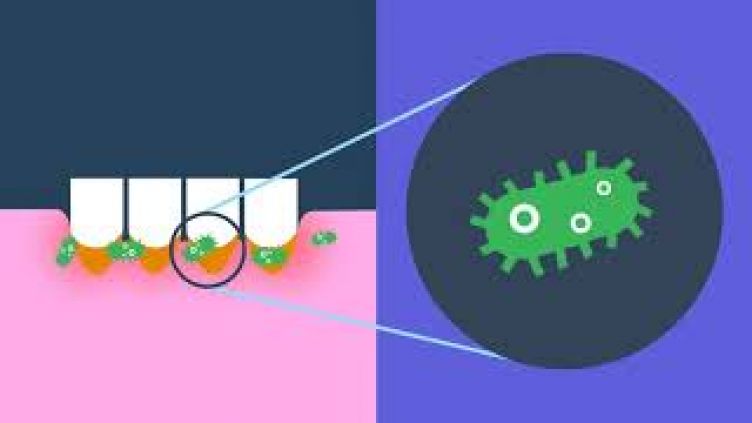
Oral Squamous Cell Carcinoma (OSCC) is a site defined subtype of Head and Neck Cancer, which is the 8th most common cancer in the UK. OSCC is increasing in incidence and the survival rate over 5 years is poor, with little improvement over the past few decades. Oral epithelial dysplasia (OED) is a precursor to OSCC and grading of OED is the gold standard to predict clinical outcome in OED. However, it is known that there is significant inter-observer variability between pathologists when grading OED, which makes grading challenging. Furthermore, assessment of early invasion in OSCC can be extremely difficult in certain cases. R-loops are DNA:RNA hybrids which occur in the genome during transcription, where RNA anneals to DNA, leaving a free-single strand of DNA (see attached picture, created with Biorender.com). R-loops are known to form during transcription, however they can also form away from the transcription site where the nascent mRNA re-anneals to the DNA. R-loops occupy 5-10% of the genome and are recognised to regulate gene expression, as well as being a potential source of genomic instability if inappropriately regulated. R-loops can be visualised in tissue using the S9.6 antibody, which is an antibody which is identified to specifically bind DNA:RNA hybrids. High levels of R-loops have been linked to a number of diseases including cancers (such as synovial sarcoma and Embryonal Tumour with Multi-layered Rosettes (ETMR)) alongside neurodegenerative diseases. However, their role in the development of OED and OSCC remains unexplored. Preliminary data shows an increase in R-loop expression in OED and OSCC when compared to normal oral mucosa, however comparison of R-loop expression between different OED grades and OSCC types has not previously been undertaken.
Aim
The aim of this project is to determine the expression of R-loops in a cohort of oral dysplasia and OSCC cases. Specifically, expression of S9.6 by immunohistochemistry will be compared between varying OED grades, early invasive OSCC and widely invasive OSCC.
Techniques/Methods
This project will involve a number of techniques and you will gain comprehensive experience of the histopathological features of OED and OSCC, alongside extensive experience of immunohistochemistry and subsequent analysis. Specifically, S9.6 immunohistochemistry will be undertaken on a cohort of normal oral mucosa (n=5), varying grades of OED (n=15), early invasive OSCC (n=5) and widely invasive OSCC (n=5). S9.6 immunohistochemistry has been previously undertaken in the laboratory and is already optimised. Following immunohistochemistry, the slides will either be digitally scanned or photomicrographs taken and quantitatively analysed in QuPath to determine the S9.6 expression in the tumour and surrounding immune infiltrate for each of the cases. Statistical analysis will then be undertaken to compare S9.6 expression between groups and expression will be correlated to clinical outcomes for the OED and OSCC cases.
References
- Bankhead, Peter, Maurice B. Loughrey, José A. Fernández, Yvonne Dombrowski, Darragh G. McArt, Philip D. Dunne, Stephen McQuaid, et al. 2017. “QuPath: Open Source Software for Digital Pathology Image Analysis.” Scientific Reports 2017 7:1 7 (1): 1–7.
- https://doi.org/10.1038/s41598-017-17204-5.
- Crossley, Madzia P., Michael Bocek, and Karlene A. Cimprich. 2019. “R-Loops as Cellular Regulators and Genomic Threats.” Molecular Cell. Cell Press.
- https://doi.org/10.1016/j.molcel.2019.01.024.
- Kujan, Omar, Ammar Khattab, Richard J. Oliver, Stephen A. Roberts, Nalin Thakker, and Philip Sloan. 2007. “Why Oral Histopathology Suffers Inter-Observer Variability on Grading Oral Epithelial Dysplasia: An Attempt to Understand the Sources of Variation.” Oral Oncology 43 (3): 224–31. https://doi.org/10.1016/J.ORALONCOLOGY.2006.03.009.
- Mahmood, Hanya, Mike Bradburn, Nasir Rajpoot, Nadim Mohammed Islam, Omar Kujan, and Syed Ali Khurram. 2022. “Prediction of Malignant Transformation and Recurrence of Oral Epithelial Dysplasia Using Architectural and Cytological Feature Specific Prognostic Models. Modern Pathology 2022 35:9 35 (9): 1151–59. https://doi.org/10.1038/s41379-022-01067-x.
- Wells, James P., Justin White, and Peter C. Stirling. 2019. “R Loops and Their Composite Cancer Connections.” Trends in Cancer. Cell Press. https://doi.org/10.1016/j.trecan.2019.08.006.
External Partner Details: Not applicable
Use of human tissue: Yes
HBV vaccination required: Yes
Ethics Approval: Yes, in place
For further information please contact Dr H Crane at h.crane@sheffield.ac.uk
- Environmental bacteriophage as sources for novel antimicrobials to combat AMR pathogens
-
Supervisors: Prof G Stafford (Academic) K Prysanskowa, A Alrafaie (Day-to-day)
Background
Bacteriophages are viruses that specifically target bacteria. They have long been considered as potential antimicrobials for the treatment of incurable AMR infections. These viruses are likely the most common organisms on the planet and occur wherever bacteria live.
Aims
- Isolation of novel bacteriophage from the environment targetting clinical isolates of bacteria of the genera Staphylococcus and Enterococcus from Diabetic foot ulcers
- Characterisation of any isolated phage using TEM (and potentially genome sequencing)
- Characterisation of existing phage in the Sheffield collection
Techniques/Methods
- Microbial culture
- DNA extraction
- Transmission electron microscopy
- AMR assays
References
- Al-Zubidi et al., 2019. doi: 10.1128/IAI.00512-19.
- Brives, C., Pourraz, J Palgrave Commun 6, 100 (2020). https://doi.org/10.1057/s41599-020- 0478-4
- Katherine E. Macdonald, et al PLOS https://doi.org/10.1371/journal.pone.0243947 • R. Fish et al Journal of Wound Care Vol. 25, No. Sup7 https://doi.org/10.12968/jowc.2016.25.Sup7.S27
External Partner Details: Not applicable
Use of human tissue: No
HBV vaccination required: No
Ethics Approval: Not required
For further information please contact Prof G Stafford on g.stafford@sheffield.ac.uk
- Establishing Early Invasion in SCC
-
Supervisors: Prof A Khurram, Dr Walsh and Dr Crane
Background
Oral squamous cell carcinoma (OSCC) is amongst the top ten most common cancers in the world and is frequently preceded by epithelial dysplasia. In small and tangentially sectioned biopsies, it can be extremely challenging to establish a diagnosis of early invasion resulting in equivocal reports.
Periodic acid–Schiff (PAS) staining is used to detect polysaccharides (e.g. glycogen), as well as glycoproteins, glycolipids and mucins in tissues. Staining for PAS and PAS-D is consistently seen in intact epithelial basement membranes. Similarly, Collagen-IV is a key component of the basement membrane and its breach is essential for invasion. However, the s use to confirm the presence of invasion in early invasive OSCC or tangentially sectioned and small specimens has not been reported to date.
Aims
The findings from this study have the potential to aid challenging diagnoses and for rapid clinical use in laboratories across the world.
Techniques/Methods
This project will involve retrospective evaluation of a cohort of biopsy specimens including small and cross-cut specimens and cases where it was not possible establish definite invasion. Normal oral mucosa, dysplastic and known OSCC cases will be included for comparison. Staining for H&E, PAS and PAS-D will be carried out and assessed. Staining for Collagen-IV (a key component of the basement membrane) will also be performed and assessed.
References
- https://pubmed.ncbi.nlm.nih.gov/26170698/
- https://pubmed.ncbi.nlm.nih.gov/23771372/
- https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4601410/
External Partner Details: Not Applicable
Use of human tissue: Yes
HBV vaccination required: No
Ethics Approval: Yes, in place
For further information please contact Prof A Khurram on s.a.khurram@sheffield.ac.uk
- Characterisation of immune response in oral epithelial dysplasia
-
Supervisors: Prof A Khurram and Dr H Mahmood
Background
Oral epithelial dysplasia (OED) is a precursor to oral squamous cell carcinoma (OSCC) which is one of the leading cancers in the world with increasing frequency and associated mortality. At present, the gold standard for OED diagnosis is histological grading, however numerous studies have shown this to be an unreliable predictor of prognosis with significant inter and intra observer variations. Our previous work has shown a correlation of histology specific features and immune cells (lymphocytes) with malignant transformation.
Aims
This project aims to explore this further by characterising the immune cell types.
Techniques/Methods
A cohort of OEDs with 5 year follow up will be selected from the departmental archive. Immunohistochemistry for lymphocyte markers (including B and T cells markers and activation markers such as CD3, CD19, CD20, CD25, CD30, CD4, CD8, CD45, CD69, PD-L1 and HLA-DR) will be carried out followed by quantification and analysis using a bio-image analysis software. Immune cell score will be correlated with OED grade and transformation risk using statistical analyses. This project has the potential to reveal novel insights into the role of the immune infiltrate in OED and aid prognosis prediction.
References
- Bashir RMS, Shephard AJ, Mahmood H, Azarmehr N, Raza SEA, Khurram SA, Rajpoot NM. A digital score of peri-epithelial lymphocytic activity predicts malignant transformation in oral epithelial dysplasia. J Pathol. 2023 Aug;260(4):431-442. doi: 10.1002/path.6094.
- Mahmood H, Bradburn M, Rajpoot N, Islam NM, Kujan O, Khurram SA. Prediction of malignant transformation and recurrence of oral epithelial dysplasia using architectural and cytological feature specific prognostic models. Mod Pathol. 2022 Sep;35(9):1151-1159. doi: 10.1038/s41379-022-01067-x.
- Hankinson PM, Mohammed-Ali RI, Smith AT, Khurram SA. Malignant transformation in a cohort of patients with oral epithelial dysplasia. Br J Oral Maxillofac Surg. 2021 Nov;59(9):1099-1101. doi: 10.1016/j.bjoms.2021.02.019.
External Partner Details: Not Applicable
Use of human tissue: Yes
HBV vaccination required: Yes
Ethics Approval: Yes, in place
For further information please contact Prof A Khurram on s.a.khurram@sheffield.ac.uk
- Conductive Electrospun Fibres for influencing Mesenchymal Stem Cell (MSC) fate
-
Supervisors: Dr T Paterson and Dr I Ortega
Background
Regenerative dentistry constitutes a critical component of contemporary dental research. The manipulation of mesenchymal stem cell (MSC) behaviour, specifically their proliferation and differentiation into bone, is one of the present challenges in this field. Many factors are known to influence MSC fate, including the chemical and mechanical properties of substrates. Recently, electroactive materials have demonstrated potential in promoting stem cell growth and differentiation, opening avenues for novel regenerative treatments.
Aims
The primary objective of this project is to evaluate the impact of conductive electrospun fibres, specifically those containing graphite particles, on the proliferation and differentiation of MSCs.
Techniques/Methods
This project will require the manufacturing of electrospun poly-caprolactone (PCL) fibres incorporated with conductive graphite particles, which will serve as the substrate for MSC culture. These scaffolds will be characterised mechanically and electronically. Comparative analysis will be performed between MSCs cultured on these electroactive membranes and those on control membranes, observing changes in MSC morphology, proliferation, and differentiation. This project will develop techniques from material science, cell culture, microscopy, and potentially molecular biology.
References
- Paterson TE, Beal SN, Santocildes-Romero ME, Sidambe AT, Hatton PV, Asencio IO. Selective laser melting–enabled electrospinning: Introducing complexity within electrospun membranes. Proceedings of the Institution of Mechanical Engineers, Part H: Journal of Engineering in Medicine. 2017;231(6):565-574. doi:10.1177/0954411917690182
- Hardy, J.G., Villancio-Wolter, M.K., Sukhavasi, R.C., Mouser, D.J., Aguilar, D., Jr., Geissler, S.A., Kaplan, D.L. and Schmidt, C.E. (2015), Electrical Stimulation of Human Mesenchymal Stem Cells on Conductive Nanofibers Enhances their Differentiation toward Osteogenic Outcomes. Macromol. Rapid Commun., 36: 1884-1890. https://doi.org/10.1002/marc.201500233 3.
- Paterson, T. E., N. Hagis, D. Boufidis, Q. Wang, S. R. Moore, A. C. Da Silva, R. L. Mitchell, J. J. P. Alix, and I. R. Minev. "Monitoring of hand function enabled by low complexity sensors printed on textile." Flexible and Printed Electronics 7, no. 3 (2022): 035003. https://doi.org/10.1088/2058-8585/ac7dd1
External Partner Details: Not Applicable
Use of human tissue: No
HBV vaccination required: No
Ethics Approval: Not required
For further information please contact Dr T Paterson on t.paterson@sheffield.ac.uk
- Developing and Evaluating Conductive Electrospun Sensors for Jaw Movement Tracking
-
Supervisors: Dr T Paterson and Dr I Ortega
Background
The tracking and monitoring jaw movements can provide vital information about the health of the oral cavity, jaw joint disorders and sleep apnea, among other conditions. High precision, cost effective tracking solutions are in demand for both clinical and at-home monitoring. While electrospun fibres are being extensively researched for their applications in various fields, their potential use as sensors in dental applications still needs to be explored.
Aims
This project aims to develop a conductive electrospun sensor using poly-caprolactone (PCL) and Poly(3,4-ethylenedioxythiophene) (PEDOT) for tracking jaw movements with high precision.
Techniques/Methods
The project will employ electrospinning to create PCL fibres, which will then be coated with PEDOT. The conductive properties of the resultant material will be characterised, as will its electrical response to strain. The sensor will be utilised for tracking jaw movements, and its precision and reliability will be evaluated on a model. The project will involve materials science, electrical engineering, and data analysis techniques.
References
- Paterson, T. E., N. Hagis, D. Boufidis, Q. Wang, S. R. Moore, A. C. Da Silva, R. L. Mitchell, J. J. P. Alix, and I. R. Minev. "Monitoring of hand function enabled by low complexity sensors printed on textile." Flexible and Printed Electronics 7, no. 3 (2022): 035003. https://doi.org/10.1088/2058-8585/ac7dd1
- Huang, J., Li, D., Zhao, M., Mensah, A., Lv, P., Tian, X., Huang, F., Ke, H., Wei, Q., Highly Sensitive and Stretchable CNT-Bridged AgNP Strain Sensor Based on TPU Electrospun Membrane for Human Motion Detection. Adv. Electron. Mater. 2019, 5, 1900241. https://doi.org/10.1002/aelm.201900241
- Tondera, C., Akbar, T. F., Thomas, A. K., Lin, W., Werner, C., Busskamp, V., Zhang, Y., Minev, I. R., Highly Conductive, Stretchable, and Cell-Adhesive Hydrogel by Nanoclay Doping. Small 2019, 15, 1901406. https://doi.org/10.1002/smll.201901406
External Partner Details: Not Applicable
Use of human tissue: No
HBV vaccination required: No
Ethics Approval: Not required
For further information please contact Dr T Paterson on t.paterson@sheffield.ac.uk
- Impact of External Cooling on Neuronal Cells: An Investigation Using a Prototype Cooling Platform
-
Supervisors: Dr T Paterson and Dr M De Felice
Background
The tracking and monitoring jaw movements can provide vital information about the health of the oral cavity, jaw joint disorders and sleep apnea, among other conditions. High precision, cost effective tracking solutions are in demand for both clinical and at-home monitoring. While electrospun fibres are being extensively researched for their applications in various fields, their potential use as sensors in dental applications still needs to be explored.
Aims
This project aims to develop a conductive electrospun sensor using poly-caprolactone (PCL) and Poly(3,4-ethylenedioxythiophene) (PEDOT) for tracking jaw movements with high precision.
Techniques/Methods
The project will employ electrospinning to create PCL fibres, which will then be coated with PEDOT. The conductive properties of the resultant material will be characterised, as will its electrical response to strain. The sensor will be utilised for tracking jaw movements, and its precision and reliability will be evaluated on a model. The project will involve materials science, electrical engineering, and data analysis techniques.
References
1. Paterson, T. E., N. Hagis, D. Boufidis, Q. Wang, S. R. Moore, A. C. Da Silva, R. L. Mitchell, J. J. P. Alix, and I. R. Minev. "Monitoring of hand function enabled by low complexity sensors printed on textile." Flexible and Printed Electronics 7, no. 3 (2022): 035003. https://doi.org/10.1088/2058-8585/ac7dd1
2. Huang, J., Li, D., Zhao, M., Mensah, A., Lv, P., Tian, X., Huang, F., Ke, H., Wei, Q., Highly Sensitive and Stretchable CNT-Bridged AgNP Strain Sensor Based on TPU Electrospun Membrane for Human Motion Detection. Adv. Electron. Mater. 2019, 5, 1900241. https://doi.org/10.1002/aelm.201900241
3. 3.Tondera, C., Akbar, T. F., Thomas, A. K., Lin, W., Werner, C., Busskamp, V., Zhang, Y., Minev, I. R., Highly Conductive, Stretchable, and Cell-Adhesive Hydrogel by Nanoclay Doping. Small 2019, 15, 1901406. https://doi.org/10.1002/smll.201901406
External Partner Details: Not Applicable
Use of human tissue: No
HBV vaccination required: No
Ethics Approval: Not required
For further information please contact Dr T Paterson on t.paterson@sheffield.ac.uk
What our students say
More information about the BMedSci (Intercalated degree)
- Programme aims
-
- To provide an enhanced knowledge and understanding of biomedical research and its methods
- To develop skills in research evaluation, communication and ethics
- To allow you to apply the above through an extended research project
- Programme outcomes
-
- The place of research in dentistry
- Current dental research and its methods
- The conduct of research in accordance with correct research methodologies and procedures
- The importance of conducting research in accordance with up-to-date ethical guidelines and policies
- The fundamental principles of designing research projects and protocols
- Academic and intellectual skills
-
- Design a research project in accordance with appropriate research methodologies and ethical principles
- Exercise independent judgment and critical thinking
- Apply basic statistical methods to data evaluation and interpretation
- Present work orally and in writing to an academic audience
- Where their project requires it, carry out practical experiments and tasks in a laboratory setting in accordance with health and safety guidelines
- Produce a well-structured and substantial dissertation to present the results of their research project
- Conduct an extensive literature review using relevant sources
- Transferable skills
-
- Apply good time-management skills to structure their work and meet deadlines
- Effectively use a wide range of IT packages for a variety of tasks
- Work independently on a project
- Display good written and oral communication skills
- Understand and apply basic statistical methods
- Self-direct their learning
- Taught element
-
If you undertake the BMedSci, you are required to attend a taught element of the programme. The taught element consists of the following designed to support the research experience:
- Medical Research Skills
- Ethics for Medicine
- Applied Statistics for Medical and Health
Medical Research Skills
This module will run over the entire length of the course with activities occurring at least once a month. The aim of the module is to provide training in key research skills, including critical appraisal of research publications, information literacy, hypothesis testing, experimental design, scientific writing, poster generation, and oral presentation skills. The module will be assessed by a critical appraisal of a publication related to the student’s research project.
Ethics for Medicine
The ethics module will run over the first 8 weeks of the BMedSci and includes lectures, tutorials, practical sessions and online training. The module aims to help students to form their own opinions and provides them with the tools and knowledge that they may need to discuss and deal with ethical issues in medicine, including good clinical practice training. The module will be assessed by a group ethics presentation and a short reflective essay.
Applied Statistics for Medical and Health researchers
The Statistics module will run over 9 weeks and will comprise of lectures, tutorials and practical sessions, including guidance on SPSS. The module will cover fundamental statistical concepts, and both simple statistical methods and the more widely used advanced methods of multiple linear regression and survival analysis. It will be an applied module, equipping students with the knowledge and skills necessary to analyse a study to answer specific research questions; to understand and critically appraise the literature and to present research findings in a suitable fashion. The lectures will be delivered online with students expected to attend for tutorial sessions. The module will be assessed by analysis of a quantitative data set using SPSS, with students answering a number of research questions in a structured format
- Summative assessment
-
The three elements comprising the summative assessment of the BMedSci are:
- Medical Research Skills worth 10% of the final mark
- Ethics for Medicine worth 10% of the final mark
- Applied Statistics for Medical and Health worth 10% of the final mark
- Dissertation worth 70% of the final mark
- External employment
-
In accordance with the guidelines set out in the University’s Students’ Charter, students are reminded that they are advised to undertake no more than 16 hours of external work per week in addition to that required by their programme of study.
- Research within the School of Clinical Dentistry
-
New accordion content
- Prizes awarded to BMedSci Graduates
-
Sheffield Prizes: British Society for Oral and Dental Research Junior Colgate Prize:
2011, 2012,2013, 2015, 2017, 2018, 2019
2011 Dominic Smith;
Tom Evans 2nd2012 Hannah Crane
2013 Joe White
2015 Paul Hankinson
2016 Chris Platais
2017 Heather Wallis
2018 Zahra Kidy
2019 Alice Rigby
IADR Hatton Prize (international)
2018 Heather Wallis - runner upINSPIRE 2016:
Jamie Hall Oral Presentation,
Heather Wallis Poster PrizeRoyal Society of Medicine, Colyer Prize 2020
Zahra KidyStudents have also been invited to present their work at other national and international meetings:
Applied and Integrated Medical Sciences intercalators conference, Bristol
BDA/Dentsply student scientist award, Wicklow, Ireland and London
IADR: Seattle, USA; Brazil
PER/ IADR Helsinki, Finland, Sept 2012
IADR Cape Town, South Africa, Summer 2014
INSPIRE Conference, London, October 2015
ABAOMS Conference, Sheffield, November 2015
INSPIRE Conference, Bristol, November 2018
IADR London, July 2018
IADR Vancouver, Canada, 2019
IADR Virtual meeting, 2020
Frequently asked questions
- What sort of work does the BMedSci involve?
-
This will really depend on the type of project you undertake. Everyone will do a statistics and ethics module, which is led by the medical school, and is assessed via coursework and a group presentation. The rest of your time is up to you to structure and will be very varied. Some projects will have lots of time spent in the different laboratories working with PhD students and also independently.
- Will I be eligible for student finance during my BMedSci?
-
You will still be able to get student finance for a maintenance loan and to cover fees. However, the NHS bursary means even if you do the BMedSci you only need to cover 4 years of fees. This means that you’ll pay the fees during your BMedSci year but not during 4th or 5th BDS. There are also bursaries to apply for and whilst funding can’t be guaranteed there is often money available towards fees and /or living costs.
- Will I deskill clinically during my BMedSci?
-
During your BMedSci you will still continue to have restorative dentistry clinics around once a week. Many previous students actually felt more confident clinically after their BMedSci and were also in the position of having done extra work to count towards their targets.
- Will it be difficult fitting into a new year group?
-
During your BMedSci clinics you will join in with the year group below after the Easter holiday which is a good way to get to know people. You also end up with friends in both your original year and new year groups.
- Is it OK if I’ve not had any research experience before?
-
Yes, most people will not have any research experience before starting their BMedSci. Supervisors are really helpful as are the PhD students in the different research groups. You also have the statistics and ethics teaching which can help too. The BMedSci is a great way to work out whether further research or an academic role would suit you in the future.
- Why is it worth doing a BMedSci?
-
It is an interesting course which is very different to the BDS degree. It gives you chances to present your work at conferences and potentially become published. You also develop useful skills including independent working and an understanding of different scientific techniques.
If you have any further questions, you can complete our enquiry form.
For more information about the BMedSci (Intercalated degree), please contact:
Programme Lead
Professor Fiona Boissonade
f.boissonade@sheffield.ac.uk
0114 215 9314
Administrative Officer
Leyna Wright
leyna.wright@sheffield.ac.uk
0114 215 9304