X-ray Crystallography
A suite of protein crystallography laboratories, featuring robotic crystallization, dedicated crystal storage, automated crystal viewing, state of the art X-ray data collection facilities, high speed data processing and molecular graphics complements our fully specified molecular biology and protein purification laboratory, where the biological samples to be studied are prepared.
Our suite of facilities
Protein preparation
We normally clone our desired protein into an overexpression plasmid, transfect the host (usually E.coli) and grow large quantities of cells in shaker flasks or fermentors. The recombinant protein is purified using a variety of chromatographic steps, such as ion exchange, size exclusion, HPLC, FPLC and gel filtration.
Crystallisation robot
For any given protein there is currently no way to predict the set of conditions that will give rise to single, well diffracting crystals. Thus many hundreds of conditions are tried on a single sample, using a sparse matrix method, varying pH, buffer, precipitant and salt concentration, and a variety of additives such as substrates or inhibitors.
Once an initial hit is obtained, many follow up crystallization trials are undertaken, screening in fine steps around the initial conditions.
We have a Matrix Hydra II PlusOne crystallization robot that can set down hundreds of different conditions in a morning, using 200nl protein samples. Thus from a small initial sample of protein, the chances of obtaining crystals are maximised.
Crystal hotel
One of the crystallization variables is temperature. Most of our samples are stored in a dedicated fixed temperature (17C) crystal hotel, to minimise environmental fluctuations. We also have smaller, vibration free incubators to probe differing temperatures, (usually 4C) for certain samples.
Check for crystals
Screening the many hundreds of different crystallization trials for any given protein for possible hits is a crucial part of the crystallization experiment. A semi-automatic plate reader and image capture device facilitates identification of crystals.
X-ray facility
Crystals are screened and data collected on the in-house X-ray facility. X-rays are produced using a Rigaku MicroMax 007 micro-focus copper rotating anode generator running at 40kV, 20mA. Two data collection stations are mounted on the generator.
For each station the X-rays are focussed onto the crystal using confocal multilayer optics. One station uses Osmic Varimax MaxFlux optics to optimise the beam intensity at the crystal, with the other station optimised for crystals with long unit cells using the Osmic Varimax HighRes optics.
For each data collection station, the crystal is cooled to 100K with an Oxford Cryosystems Cryostream nitrogen cooler, and data accumulated using a MarResearch MAR345 image plate detector system.
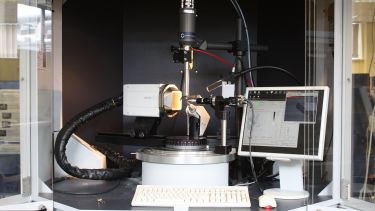
X-ray station
Crystals are screened and data collected on the in-house X-ray facility. X-rays are produced using a Rigaku MicroMax 007 micro-focus copper rotating anode generator running at 40kV, 20mA. Two data collection stations are mounted on the generator.
For each station the X-rays are focussed onto the crystal using confocal multilayer optics.
One station uses Osmic Varimax MaxFlux optics to optimise the beam intensity at the crystal, with the other station optimised for crystals with long unit cells using the Osmic Varimax HighRes optics.
For each data collection station, the crystal is cooled to 100K with an Oxford Cryosystems Cryostream nitrogen cooler, and data accumulated using a MarResearch MAR345 image plate detector system.
The process
Having first identified, cloned, overexpressed and purified the target protein of interest, a fully automatic Matrix Hydra II PlusOne crystallisation robot is used to test many hundreds of possible crystallisation conditions.
Trials are stored in a dedicated, temperature stable crystal hotel, and checked using a semi-automatic plate reader for possible crystal hits.
Crystals are screened and data collected on the in-house X-ray facility. X-rays are produced using a Rigaku MicroMax 007 micro-focus copper rotating anode generator running at 40kV, 20mA. Two data collection stations are mounted on the generator.
For each station the X-rays are focussed onto the crystal using confocal multilayer optics. One station uses Osmic Varimax MaxFlux optics to optimise the beam intensity at the crystal, with the other station optimised for crystals with long unit cells using the Osmic Varimax HighRes optics.
For each data collection station, the crystal is cooled to 100K with an Oxford Cryosystems Cryostream nitrogen cooler, and data accumulated using a MarResearch MAR345 image plate detector system. Data are processed, structures determined and molecular structures viewed using a cluster of Unix, Linux and OSX computers.
Grants
Funded through grants from:
- BBSRC
- EPSRC
- Wellcome Trust
- The Royal Society
- Wolfson Foundation
- EU
- NEDO
