Dr Tennore Ramesh
DVM, PhD
Neuroscience, School of Medicine and Population Health
Non-Clinical Lecturer
+44 114 222 2246
Full contact details
Neuroscience, School of Medicine and Population Health
Sheffield Institute for Translational Neuroscience (SITraN)
385a Glossop Road
Sheffield
S10 2HQ
- Profile
-
- Dec 2008-Present Non-Clinical Lecturer, Academic Neurology, University of Sheffield, UK.
- 2004-2008 Founder, PALS Fund for ALS Drug Discovery, Ohio State University. Research scientist, Ohio State University, Columbus, OH
- 2000-8/2003 Founding Scientist and Chief Scientific Officer, ALS-TDF, Newton, MA, USA
- 1999- 2000 Scientist, Toxico and Pharmacogenomics, CuraGen Corporation, New Haven, CT, USA
- 1998-1999 Post-Doctoral Research Fellow, Human Genetics, University of Michigan, Ann Arbor, MI, USA
- 1996-1998 Post-Doctoral Research Fellow, Neurobiotechnology Center, Ohio State University, Columbus, OH, USA
- 1992-1996 PhD, Microbiology and Immunology, University of Kentucky, Lexington, KY, USA
- 1990-1992, MS, Animal Sciences, University of Kentucky, Lexington, KY
- 1982-1988 B.V.Sc (D.V.M), Madras Veterinary College, India
- Research interests
-
Amyotrophic lateral sclerosis/Motor neuron disease (ALS/MND) is an adult onset motoneuron degenerative disease with a lifetime risk of ~1/1000. Approximately 80% of the cases are fatal within five years of diagnosis. There is no cure and only one FDA approved therapy, Riluzole has a minor effect on the progression of the disease.
Mutations in genes such as SOD1, TDP-43, FUS, ANG, and VAPB also cause some forms of ALS although most forms of ALS is sporadic in nature. Despite the identification many genes causing ALS the exact mechanism of motor neuron toxicity is unclear, although a variety of mechanisms have been postulated.
Identifying the upstream events that result in toxicity is critical to impact the disease process. Protein misfolding and cellular inclusions are a common theme in many neurodegenerative diseases including ALS. How protein misfolding contributes to toxicity, if protein inclusions are protective or detrimental is presently unknown.
In most cases the accumulation of mutant proteins are not universally present in all cells but are restricted to specific cell types and also to specific regions in the CNS affected in the disease. Understanding the cause and mechanism of this toxic process is one of the focuses of my research. To understand disease process in this complex environment requires complex systems such as mice and more recently zebrafish.
My lab utilises the power of mice and fish to study the pathogenic processes involved in neuronal death. Many transgenic models of ALS have been developed and have given valuable insight into the cellular players involved in disease process. My lab recently developed a transgenic zebrafish model of ALS with mutation in the sod1 gene.
Transgenic sod1 zebrafish carrying mutant sod1 develop disease that is similar to that seen in mice and human. Additionally, these transgenic fish show early embryonic readout of mutant sod1 induced cellular stress response as early as 24 hours post-fertilization allowing us to study disease in these microscopic stages.
Zebrafish are tropical fishes who are optically transparent in early embryonic and larval stages. Organogenesis is complete and freely swimming larvae hatch as early as 72 hours post-fertilization, providing a powerful tool to study tissue specific changes in diseased animals. Using GFP transgenic lines, we can visualize motoneurons and other spinal neurons cells in living animals.
We can also analyze cell fate, axonal outgrowth and morphology, synapses (formation, maintenance, and activity), and motor behaviour. We can easily generate genetic mosaics to determine the autonomy of mutant sod1 and examine the cell autonomy of the mutant gene.
This will allow us to readily address what cells contribute to the disease and the effect of mutant cells on wild-type motoneurons, an area, which is still in need of investigation. We employ molecular, cell biology, behavioural and genetic techniques to unravel the toxic mechanisms involved in ALS pathogenesis.
Eventually, our goal is to test genes and drugs that can modify disease process to develop new therapies to treat ALS/MND.
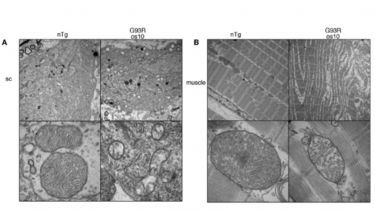
Figure 1: Pathological changes were observed in G93R os10 spinal cord and muscle. A) Electron microscopy of a spinal cord motoneuron from nTg (left) and G93R os10 (right) adult end-stage fish. Numerous vacuolated mitochondria were observed in G93R os10 motoneurons (top, 9,300 X) and were readily apparent at 68,000 X (bottom). B) Electron microscopy also revealed severe abnormalities in G93R os10 muscle (top, 6,800 X). Mitochondria in G93R os10 muscle also appeared to be affected, both in number and integrity, and cristae appeared to be reduced in number and lacked the normal organisation (bottom 68,000). 223 x 110mm (300 x 300 DPI).
- Publications
-
Journal articles
- Animal models of multiple sclerosis: From rodents to zebrafish.. Multiple Sclerosis Journal, 25(3), 306-324. View this article in WRRO


- Mutations in the Glycosyltransferase Domain of GLT8D1 Are Associated with Familial Amyotrophic Lateral Sclerosis. Cell Reports, 26(9), 2298-2306.e5. View this article in WRRO


- Stable transgenic C9orf72 zebrafish model key aspects of the ALS/FTD phenotype and reveal novel pathological features. Acta Neuropathologica Communications, 6(1). View this article in WRRO


- The effect of hyperglycemia on neurovascular coupling and cerebrovascular patterning in zebrafish. Journal of Cerebral Blood Flow and Metabolism. View this article in WRRO


- ZNStress: A high-throughput drug screening protocol for identification of compounds modulating neuronal stress in the transgenic mutant sod1G93R zebrafish model of amyotrophic lateral sclerosis. Molecular Neurodegeneration, 11(1), 56-56. View this article in WRRO


- Deficiency in the mRNA export mediator Gle1 impairs Schwann cell development in the zebrafish embryo. Neuroscience, 322, 287-297. View this article in WRRO


- A zebrafish model exemplifies the long preclinical period of motor neuron disease.. J Neurol Neurosurg Psychiatry, 85(11), 1288-1289. View this article in WRRO


- Abnormalities in whisking behaviour are associated with lesions in brain stem nuclei in a mouse model of amyotrophic lateral sclerosis.. Behav Brain Res, 259, 274-283. View this article in WRRO


- A new zebrafish model produced by TILLING of SOD1-related amyotrophic lateral sclerosis replicates key features of the disease and represents a tool for in vivo therapeutic screening.. Dis Model Mech, 7(1), 73-81.


- Tardbpl splicing rescues motor neuron and axonal development in a mutant tardbp zebrafish.. Hum Mol Genet, 22(12), 2376-2386. View this article in WRRO


- Axonal Transport Defects in a Mitofusin 2 Loss of Function Model of Charcot-Marie-Tooth Disease in Zebrafish.. PLoS One, 8(6), e67276. View this article in WRRO


- Early interneuron dysfunction in ALS: insights from a mutant sod1 zebrafish model.. Ann Neurol, 73(2), 246-258. View this article in WRRO


- A genetic model of amyotrophic lateral sclerosis in zebrafish displays phenotypic hallmarks of motoneuron disease.. Dis Model Mech, 3(9-10), 652-662.


- Report of the European Zebrafish Principal Investigator Meeting in Padua, Italy, March 18-22, 2010. Zebrafish, 7(3), 305-310.


- Alzheimer research forum live discussion: Mice on trial? issues in the design of drug studies. Journal of Alzheimer's Disease, 16(1), 197-205.


- Determination of clodronate content in liposomal formulation by capillary zone electrophoresis.. J Pharm Biomed Anal, 31(5), 929-935.


- Novel trends in orphan market drug discovery: amyotrophic lateral sclerosis as a case study.. Front Biosci, 7, c83-c96.


- Novel trends in orphan market drug discovery: amyotrophic lateral sclerosis as a case study.. Frontiers in bioscience : a journal and virtual library, 7.


- Human intrathecal transplantation of peripheral blood stem cells in amyotrophic lateral sclerosis. Journal of Hematotherapy and Stem Cell Research, 10(6), 913-915.


- Position-dependent activity of alpha -fetoprotein enhancer element III in the adult liver is due to negative regulation.. Proc Natl Acad Sci U S A, 97(20), 10890-10894.


- Integrating expression-based drug response and SNP-based pharmacogenetic strategies into a single comprehensive pharmacogenomics program. Drug Development Research, 49(1), 54-64.


- Altered anxiety and weight gain in corticotropin-releasing hormone-binding protein-deficient mice. Proceedings of the National Academy of Sciences of the United States of America, 96(20), 11595-11600.


- Ectopic expression of class I histocompatibility D-d proteins during mouse development results in neural tube defects. TRANSGENICS, 2(4), 391-401.


- Increased anxiety in corticotropin releasing hormone-binding protein-deficient mice. BRAIN RES, 809(1), A23-A24.


- Individual mouse alpha-fetoprotein enhancer elements exhibit different patterns of tissue-specific and hepatic position-dependent activities.. Mol Cell Biol, 15(9), 4947-4955.


- Individual mouse α-fetoprotein enhancer elements exhibit different patterns of tissue-specific and hepatic position-dependent activities. Molecular and Cellular Biology, 15(9), 4947-4955.


- Rapamycin, a potent immunosuppressive drug, causes programmed cell death in В lymphoma cells. Transplantation, 60(3), 264-270.


Conference proceedings papers
- 11.30 Mutations in the glycosyltransferase domain of GLT8D1 cause ALS. Journal of Neurology Neurosurgery & Psychiatry, Vol. 90(12) (pp e10)


- The development of a novel demyelination zebrafish model to be used for high-throughput drug screening of pro-myelinating compounds in multiple sclerosis. MULTIPLE SCLEROSIS JOURNAL, Vol. 21 (pp 431-431)


- Deficiency in Gle1, an mRNA export mediator, inhibits Schwann cell development in the zebrafish embryo. FEBS JOURNAL, Vol. 281 (pp 771-771)


- A novel alternative splicing event rescues the mutant tardbp phenotype in a zebrafish model of TDP-43 related Amyotrophic Lateral Sclerosis (ALS). NEUROLOGY, Vol. 78


- A novel alternative splicing event rescues the mutant tardbp phenotype in a zebrafish model of TDP-43 related Amyotrophic Lateral Sclerosis (ALS). NEUROLOGY, Vol. 78


- THE HEAT IS "ON" IN THE NEURONS: NEURONAL STRESS IN A SOD1 ZEBRAFISH MODEL OF MND AFFECTS NEUROMUSCULAR JUNCTION INTEGRITY AND CAUSES MUSCLE DENERVATION. JOURNAL OF NEUROLOGY NEUROSURGERY AND PSYCHIATRY, Vol. 83(3)


- Reduced GLE1 Protein Levels Cause Axonal Growth Defects in Zebrafish Motor Neurons. NEUROLOGY, Vol. 74(9) (pp A441-A441)


- Animal models of multiple sclerosis: From rodents to zebrafish.. Multiple Sclerosis Journal, 25(3), 306-324. View this article in WRRO
- Research group
-
Research projects available:
- Study of early pathological events in MND using transgenic sod1 zebrafish.
- Development of new zebrafish models of MND
- Development of new tools for study of disease progression and drug screening in mouse model of MND
Research Group:
- Mr. Alexander McGown: PhD student: Primary supervisor
- Dr. Channa Hewamadduma: PhD student: Co supervisor
Alumni:
- Natasha Redhead: BMedSci student:
- Sufana Al Mashhadi: MSc Molecular Neuroscience
- Marc Da Costa: PhD Student: Co supervisor
- Sumona Dhara: MSc in Stem Cell and Regenerative Medicine
- Fabiola Sica: MSc Molecular Neuroscience
- Grants
-
- Completed: ALS Association: Development of SOD1 zebrafish model of ALS: 2004-2007: Co-PI
- ALS Association: Development of modifier screen using transgenic sod1 zebrafish: 2007:Co-PI
- Sheffield Hospitals Charitable Trust: 2010: PI
- Fondation Thierry Latran: 2010: PI
- MNDA 2011
- Professional activities and memberships
-
Items of Esteem:
- 2003-2006 Scientific Advisory Board, Western ALS study group (WALS)
- 1998 NIH Reproductive sciences training grant fellowship
- 1996 NIH Neuropharmacology training grant Fellowship
- 1995 Best Poster award, Graduate student research day
- 1990 Graduate school Fellowship