Dr Kai Erdmann
School of Biosciences
Senior Lecturer in Molecular Cell Biology
Director of Postgraduate Research


- Profile
-
- 2012-present: Senior Lecturer, School of Biosciences, The University of Sheffield
- 2006-2012: Group leader, Ruhr-University Bochum, Germany
- 2005-2006: Research Associate, Yale University School of Medicine, USA
- 2003-2005: Feodor Lynen Fellow (Alexander von Humboldt foundation), Yale University School of Medicine, USA
- 1998-2003: Junior group leader, Ruhr-University Bochum, Germany
- 1996-1998: Postdoctoral work, Ruhr-University Bochum, Germany
- 1993-1996: PhD, Ruhr-University Bochum, Germany
- Research interests
-
Molecular and Cellular Mechanobiology: Mechanisms of Mechanotransduction
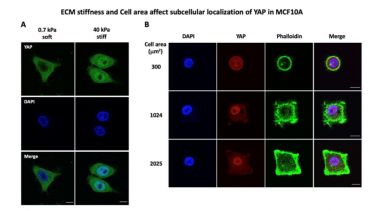
Mechanical cues like extracellular matrix stiffness, fluid flow shear stress or spatial confinement play an important role in development, cell and tissue homeostasis as well as in disease initiation or progression (e.g., cancer or fibrosis). Our group is interested in how such mechanical signals are translated into cellular biochemical changes (a process called mechanotransduction) and how these changes contribute to regulate key cellular behaviour like cell proliferation and migration. Importantly, we also investigate the role of mechanotransduction in disease progression with a special focus on its role in fibrosis and cancer. We have used the well-established mechanotransducer YAP (Figure 1) as a model to develop molecular screens to identify novel proteins with a role in mechanotransduction.
Note: We are aiming to identify proteins that are controlled in a similar manner and investigate their role in mechanotransduction.
Membrane trafficking: Regulation of receptor cell surface expression
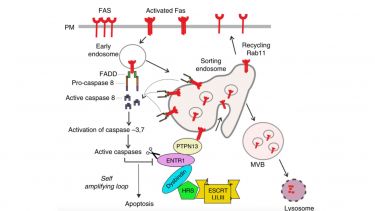
Cell surface expression levels of receptors are controlled by a tight balance of receptor endocytosis and recycling. We are investigating underpinning membrane trafficking mechanisms that regulate receptor cell surface expression. In particular we are interested in the cell surface regulation of the apoptosis receptor Fas/CD95 and have recently identified a novel crosstalk between regulation of Fas/CD95 membrane trafficking and apoptotic signalling (Figure 2). More recently we have started to investigate the effect of mechanical cues like ECM stiffness on the regulation of membrane trafficking as part of the cellular mechanoresponse.
- Publications
-
Show: Featured publications All publications
Featured publications
Journal articles
- Apoptotic signalling targets the post-endocytic sorting machinery of the death receptor Fas/CD95. Nature Communications, 10(1). View this article in WRRO


- The serologically defined colon cancer antigen-3 interacts with the protein tyrosine phosphatase PTPN13 and is involved in the regulation of cytokinesis. Oncogene.


- A structural basis for Lowe syndrome caused by mutations in the Rab-binding domain of OCRL1. EMBO Journal, 30(8), 1659-1670.


- PDZ-domain-directed basolateral targeting of the peripheral membrane protein FRMPD2 in epithelial cells. Journal of Cell Science, 122(18), 3374-3384.


- A role of the Lowe syndrome protein OCRL in early steps of the endocytic pathway.. Dev Cell, 13(3), 377-390.


- The serologically defined colon cancer antigen-3 (SDCCAG3) is involved in the regulation of ciliogenesis. Scientific Reports, 6. View this article in WRRO


All publications
Journal articles
- Protocol to identify mechanosensitive nuclear proteins using tunable actomyosin contractility and proximity biotinylation in mammalian cells. STAR Protocols, 7(1), 104288-104288.


- Mechanical control of the alternative splicing factor PTBP1 regulates extracellular matrix stiffness induced proliferation and cell spreading. iScience, 28(4). View this article in WRRO


- A 3d renal proximal tubule on chip model phenocopies Lowe syndrome and Dent II disease tubulopathy. International Journal of Molecular Sciences, 22(10).


- Development of a human primary gut-on-a-chip to model inflammatory processes. Scientific Reports, 10(1). View this article in WRRO


- Direct on-chip differentiation of intestinal tubules from induced pluripotent stem cells. International Journal of Molecular Sciences, 21(14). View this article in WRRO


- Crumbs2 mediates ventricular layer remodelling to form the spinal cord central canal. PLOS Biology, 18(3), ---.


- The binding affinity of PTPN13’s tandem PDZ2/3 domain is allosterically modulated. BMC Molecular and Cell Biology, 20(1).


- Apoptotic signalling targets the post-endocytic sorting machinery of the death receptor Fas/CD95. Nature Communications, 10(1). View this article in WRRO


- Development of a gut-on-a-chip model for high throughput disease modeling and drug discovery. International Journal of Molecular Sciences, 20(22). View this article in WRRO


- Molecular basis of class III ligand recognition by PDZ3 in murine protein tyrosine phosphatase PTPN13. Journal of Molecular Biology, 430(21), 4275-4292. View this article in WRRO


- A novel interaction between ATOH8 and PPP3CB. Histochemistry and Cell Biology, 145(1), 5-16. View this article in WRRO


- The role of the Lowe syndrome protein OCRL in the endocytic pathway. Biological Chemistry, 396(12).


- Crystal structure of the Rab binding domain of OCRL1 in complex with Rab8 and functional implications of the OCRL1/Rab8 module for Lowe syndrome.. Small GTPases, 3(2), 107-110.


- RNAi screening identifies mediators of NOD2 signaling: Implications for spatial specificity of MDP recognition. Proceedings of the National Academy of Sciences of the United States of America, 109(52), 21426-21431.


- The serologically defined colon cancer antigen-3 interacts with the protein tyrosine phosphatase PTPN13 and is involved in the regulation of cytokinesis. Oncogene.


- A structural basis for Lowe syndrome caused by mutations in the Rab-binding domain of OCRL1. EMBO Journal, 30(8), 1659-1670.


- Sequence-specific 1H, 13C, and 15N assignment of the extended PDZ3 domain of the protein tyrosine phosphatase basophil-like PTP-BL.. Biomol NMR Assign, 4(2), 199-202.


- PDZ-domain-directed basolateral targeting of the peripheral membrane protein FRMPD2 in epithelial cells. Journal of Cell Science, 122(18), 3374-3384.


- A PH domain within OCRL bridges clathrin-mediated membrane trafficking to phosphoinositide metabolism.. EMBO J, 28(13), 1831-1842.


- The protein tyrosine phosphatase-BL, modulates pancreatic β-cell proliferation by interaction with the Wnt signalling pathway. Journal of Endocrinology, 197(3), 543-552.


- The protein tyrosine phosphatase, PTP-BL, regulates pancreatic beta cell proliferation by interacting with components of the Writ signalling pathway. DIABETOLOGIA, 51, S191-S191.


- A role of the Lowe syndrome protein OCRL in early steps of the endocytic pathway.. Dev Cell, 13(3), 377-390.


- Sequence-specific (1)H, (13)C, and (15)N backbone assignment of the 28 kDa PDZ2/PDZ3 tandem domain of the protein tyrosine phosphatase PTP-BL.. Biomol NMR Assign, 1(2), 151-153.


- Dynamin and the actin cytoskeleton cooperatively regulate plasma membrane invagination by BAR and F-BAR proteins.. Dev Cell, 9(6), 791-804.


- Truncated TrkB receptor-induced outgrowth of dendritic filopodia involves the p75 neurotrophin receptor. Journal of Cell Science, 117(24), 5803-5814.


- The protein tyrosine phosphatase PTP-Basophil/Basophil-like Interacting proteins and molecular functions. European Journal of Biochemistry, 270(24), 4789-4798.


- Structure determination and ligand interactions of the PDZ2b domain of PTP-Bas (hPTP1E): Splicing-induced modulation of ligand specificity. Journal of Molecular Biology, 334(1), 143-155.


- The protein tyrosine phosphatase PTP-BL associates with the midbody and is involved in the regulation of cytokinesis.. Mol Biol Cell, 14(1), 230-240.


- EphrinB phosphorylation and reverse signaling: Regulation by Src kinases and PTP-BL phosphatase. Molecular Cell, 9(4), 725-737.


- Semaphorin4F interacts with the synapse-associated protein SAP90/PSD-95.. J Neurochem, 78(3), 482-489.


- Reduced number of functional glutamatergic synapses in hippocampal neurons overexpressing full-length TrkB receptors. Journal of Neuroscience Research, 66(3), 327-336.


- The protein kinase C-related kinase PRK2 interacts with the protein tyrosine phosphatase PTP-BL via a novel PDZ domain binding motif. FEBS Letters, 496(2-3), 101-104.


- The Adenomatous Polyposis Coli-protein (APC) interacts with the protein tyrosine phosphatase PTP-BL via an alternatively spliced PDZ domain.. Oncogene, 19(34), 3894-3901.


- Investigating TrkB receptor targeting in cortical neurons by means of a signaling-competent TrkB-EGFP fusion protein. EUROPEAN JOURNAL OF NEUROSCIENCE, 12, 3-3.


- The ephrinB-family interacts with the proteintyrosinephosphatase PTP-BL. EUROPEAN JOURNAL OF NEUROSCIENCE, 12, 180-180.


- Identification and characterization of the human orthologue of yeast Pex14p.. Mol Cell Biol, 19(3), 2265-2277.


- Interaction of the ephrinB-family with the proteintyrosine phosphatase PTPL1/PTP-BL. JOURNAL OF NEUROCHEMISTRY, 73, S125-S125.


- Ectopic expression of a chimeric colony-stimulating factor-1/TrkB-receptor promotes CSF-1-dependent survival of cultured sympathetic neurons.. Biochem Biophys Res Commun, 249(3), 891-897.


- Cloning and sequence analysis of a cDNA encoding a novel truncated form of the chicken TrkB receptor.. Gene, 149(2), 383-384.


- The serologically defined colon cancer antigen-3 (SDCCAG3) is involved in the regulation of ciliogenesis. Scientific Reports, 6. View this article in WRRO


Conference proceedings
- Protein tyrosine phosphatase PTPN13 and its interaction partner SDCCAG3 are involved in the regulation of cytokinesis. FEBS JOURNAL, Vol. 281 (pp 78-78)


- FERM and PDZ domain containing protein 2 regulates epithelial cell polariZation. FEBS JOURNAL, Vol. 281 (pp 197-197)


Preprints
- An unbiased screening approach for identifying proteins with a mechanosensitive nuclear localization, Cold Spring Harbor Laboratory.


- Mechanical control of the splicing factor PTBP1 regulates extracellular matrix stiffness-induced cell proliferation and mechanomemory, Cold Spring Harbor Laboratory.


- Crumbs2 mediates ventricular layer remodelling to form the adult spinal cord central canal, Cold Spring Harbor Laboratory.


- Apoptotic signalling targets the post-endocytic sorting machinery of the death receptor Fas/CD95. Nature Communications, 10(1). View this article in WRRO
- Research group
-
- Andrew Wood (arwood1@sheffield.ac.uk)
- Pei Li Tseng (pltseng1@sheffield.ac.uk)
- Ahmed Salem (amasalem1@sheffield.ac.uk)
- Mubarak Alaklobie (msalaklobie1@sheffield.ac.uk)
- Weiwei Sun (wsun22@sheffield.ac.uk)
Previous lab members:
- Fangyan Yu
- Shruti Sharma
- Agnieszka Skowronek
- Claire Murzeau
- Kinga Kosim
- Sindhu N Naik
- Antonio Carmona-Serrano
- Elena Naumovska
- Claudia Beaurivage
Collaborations:
- Jochen Guck Technical University, Dresden, Germany
- Grants
-
- DFG (Deutsche Forschungsgemeinschaft)
- Fritz Thyssen foundation
- EU
- Teaching activities
-
Undergraduate and postgraduate taught modules
Undergraduate
- BMS110 Research Topics in Biomedicine
- BMS301 Membrane Receptors
- BMS376 Membrane Trafficking
- Level 3 Practical and Dissertation Modules
MSc (Masters)
- BMS6082 Practical Cell Biology
- Professional activities and memberships
-
- Coordinator of the Marie Curie Initial Training Network (ITN) TRANSPOL
- Visiting scientist Yale University School of Medicine (Sept/Oct. 2011)
- Eurotrans-Bio (ETB) Award (2011) (consortium)
- Feodor Lynen postdoctoral fellow (Alexander von Humboldt foundation) (2003-2005)
- Opportunities
We advertise PhD opportunities (Funded or Self-Funded) on FindAPhD.com
For further information about these projects and how to apply at Sheffield, see our PhD Opportunities page.