Professor Marysia Placzek
School of Biosciences
Professor of Developmental Neurobiology
Full contact details
School of Biosciences
Firth Court
Western Bank
Sheffield
S10 2TN
- Profile
-
Marysia is a Wellcome Trust Investigator and Professor of Developmental Neurobiology in the School of Biosciences. She has inspired staff and students alike with her pioneering leadership.
A driving force behind one of our University’s key research facilities, Marysia directed the MRC Centre for Developmental and Biomedical Genetics for many years and oversaw its evolution into the Bateson Centre.
Her initiative and outstanding commitment to her colleagues and students contributed to the ongoing success of research in the Department and the strong focus on developmental biology, and its translation, at the University.
Marysia’s research focuses on the development of the vertebrate hypothalamus and she has earned international acclaim for her work in this field. In 2023 she was awarded the British Society for Developmental Biology Waddington Medal, in recognition of her outstanding research performance as well as services to the subject community.
In 2012 she was awarded an MRC Suffrage Science Heirloom in recognition of her achievements as a leading female scientist.
Like many women, she balances the demands of world-leading research with family commitments, and has been celebrated by The Royal Society’s ‘Parent Carer Scientist’ project.
Colleagues agree that “Marysia is not just a role model for female scientists, but for all those with an aspiration to become a leader in their field.”
- Research interests
-
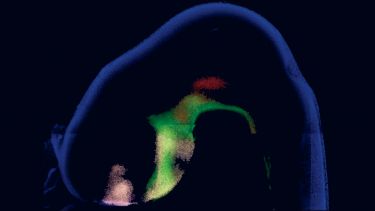
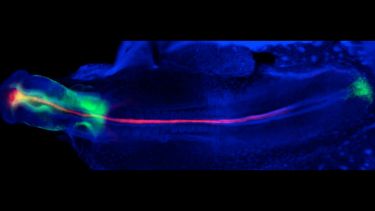
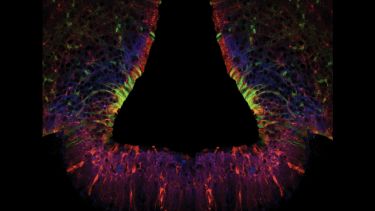
How the brain develops remains one of the greatest mysteries in science. Research in the lab focus on the development of a fascinating part of the brain - the hypothalamus – the master-controller of physical, behavioural and emotional homeostasis.
Lay summary
In the developing embryos, the hypothalamus is built with precision. This ordered assembly underlies its key role in life. Our research focuses on characterising the stem and progenitor cells that build the hypothalamus and characterising the molecular networks that direct hypothalamic morphogenesis, growth and differentiation. Our work will contribute to understanding the importance of the hypothalamus to robust long-term health and will shed light on diseases and disruptions of homeostasis.
Research summary
The functions of the hypothalamus in mediating homeostasis, and ensuring that brain and body function optimally, are well-known. By contrast, little is understood of how the hypothalamus develops. This knowledge is important, because early indications suggest that deregulation of developmental programmes may underlie complex human pathological conditions, including stress and eating disorders. Our goal is therefore to understand how the hypothalamus develops in the embryo and how the proper embryonic assembly of the hypothalamus holds the key to robust adult function.
We focus in particular on five key areas:
- The role of adjacent tissues in inducing multipotent embryonic hypothalamic progenitor cells
- The characterisation of multipotent embryonic hypothalamic progenitor cells, and identification of cues that maintain progenitor cells, or promote their differentiation
- The self-organisation and morphogenesis of hypothalamic progenitor cells through integrated growth and differentiation
- The cellular and molecular events that underlie the integrated assembly of the hypothalamo-pituitary neuraxis
- The characterization of stem/progenitor-like tanycytes in the adult hypothalamus
We use a range of animal model systems (chick, mouse, zebrafish) and combine in vivo and ex vivo approaches with state-of-the-art transcriptomic, imaging, and gain-and loss-of-function approaches.
- Publications
-
Show: Featured publications All publications
Featured publications
Journal articles
- Accurate staging of chick embryonic tissues via deep learning of salient features. Development, 150(22). View this article in WRRO


- A neuroepithelial wave of BMP signalling drives anteroposterior specification of the tuberal hypothalamus. eLife, 12. View this article in WRRO


- Single-cell analysis of early chick hypothalamic development reveals that hypothalamic cells are induced from prethalamic-like progenitors. Cell Reports, 38(3).


- PINK1 deficiency impairs adult neurogenesis of dopaminergic neurons. Scientific Reports, 11(1). View this article in WRRO


- Crumbs2 mediates ventricular layer remodelling to form the spinal cord central canal. PLOS Biology, 18(3). View this article in WRRO


- Human axial progenitors generate trunk neural crest cells in vitro. eLife, 7. View this article in WRRO


- Sonic hedgehog in vertebrate neural tube development. International Journal of Developmental Biology, 62(1-3), 221-230.


- Fgf10(+) progenitors give rise to the chick hypothalamus by rostral and caudal growth and differentiation. Development, 144(18), 3278-3288. View this article in WRRO


- Development of the Neuroendocrine Hypothalamus.. Comprehensive Physiology, 6(2), 623-643.


- α-Tanycytes of the adult hypothalamic third ventricle include distinct populations of FGF-responsive neural progenitors.. Nat Commun, 4, 2049.


Book chapters
- Development of the Neuroendocrine Hypothalamus, Masterclass in Neuroendocrinology (pp. 3-30). Springer International Publishing


Preprints
- Single-cell analysis of early chick hypothalamic development reveals that hypothalamic cells are induced from prethalamic-like progenitors, Cold Spring Harbor Laboratory.


- Crumbs2 mediates ventricular layer remodelling to form the adult spinal cord central canal, Cold Spring Harbor Laboratory.


All publications
Journal articles
- Balancing SHH and BMP/FGF10 to specify tuberal hypothalamic neurons and glia. Developmental Biology, 525, 259-269. View this article in WRRO


- Control of tuberal hypothalamic development and its implications in metabolic disorders. Nature Reviews Endocrinology, 21(2), 118-130.


- Fabrication and characterisation of random and aligned electrospun scaffolds to investigate hypothalamic stem/progenitor cell behaviour. Engineered Regeneration, 5(1), 11-20.


- A Methodology for the Enzymatic Isolation of Embryonic Hypothalamus Tissue and Its Acute or Post-Culture Analysis by Multiplex Hybridisation Chain Reaction. BIO-PROTOCOL, 13(23).


- An interview with Marysia Placzek. Development, 150(23).


- Accurate staging of chick embryonic tissues via deep learning of salient features. Development, 150(22). View this article in WRRO


- Glucocorticoid receptor regulates protein chaperone, circadian clock and affective disorder genes in the zebrafish brain. Disease Models & Mechanisms, 16(9). View this article in WRRO


- Fgf signalling triggers an intrinsic mesodermal timer that determines the duration of limb patterning. Nature Communications, 14(1). View this article in WRRO


- A neuroepithelial wave of BMP signalling drives anteroposterior specification of the tuberal hypothalamus. eLife, 12. View this article in WRRO


- Zebrafish as a model to investigate the CRH axis and interactions with DISC1. Current Opinion in Endocrine and Metabolic Research, 26.


- Loss of function of the neural cell adhesion molecule NrCAM regulates differentiation, proliferation and neurogenesis in early postnatal hypothalamic tanycytes. Frontiers in Neuroscience, 16.


- Single-cell analysis of early chick hypothalamic development reveals that hypothalamic cells are induced from prethalamic-like progenitors. Cell Reports, 38(3).


- Defining the signalling determinants of a posterior ventral spinal cord identity in human neuromesodermal progenitor derivatives. Development, 148(6). View this article in WRRO


- PINK1 deficiency impairs adult neurogenesis of dopaminergic neurons. Scientific Reports, 11(1). View this article in WRRO


- Conserved roles of Rax/rx3 genes in hypothalamus and pituitary development. The International Journal of Developmental Biology, 65(4-5-6), 195-205.


- A multiorganism pipeline for antiseizure drug discovery: Identification of chlorothymol as a novel γ‐aminobutyric acidergic anticonvulsant. Epilepsia, 61(10), 2106-2118. View this article in WRRO


- Of mitogens and morphogens : modelling Sonic Hedgehog mechanisms in vertebrate development. Philosophical Transactions of the Royal Society B: Biological Sciences, 375(1809). View this article in WRRO


- Crumbs2 mediates ventricular layer remodelling to form the spinal cord central canal. PLOS Biology, 18(3). View this article in WRRO


- Development of the basal hypothalamus through anisotropic growth. Journal of Neuroendocrinology, 31(5).


- 1.4 Disrupted-in-Schizophrenia-1 is essential for normal hypothalamic-pituitary-interrenal (HPI) axis function. ESPE Yearbook of Paediatric Endocrinology, 15. View this article in WRRO


- Human axial progenitors generate trunk neural crest cells in vitro. eLife, 7. View this article in WRRO


- Sonic hedgehog in vertebrate neural tube development. International Journal of Developmental Biology, 62(1-3), 221-230.


- Fgf10(+) progenitors give rise to the chick hypothalamus by rostral and caudal growth and differentiation. Development, 144(18), 3278-3288. View this article in WRRO


- Disrupted-in-schizophrenia-1 is essential for normal hypothalamic-pituitary-interrenal (HPI) axis function. Human Molecular Genetics, 26(11), 1992-2005. View this article in WRRO


- Rx3 and Shh direct anisotropic growth and specification in the zebrafish tuberal/anterior hypothalamus. Development, 143(14), 2651-2663.


- Development of the Neuroendocrine Hypothalamus. Comprehensive Physiology, 6(2), 623-643.


- Development of the Neuroendocrine Hypothalamus.. Comprehensive Physiology, 6(2), 623-643.


- ProNodal acts via FGFR3 to govern duration of Shh expression in the prechordal mesoderm. Development, 142(22), 3821-3832.


- Corrigendum to "Axon guidance effect of classical morphogens Shh and BMP7 in the hypothalamo-pituitary system" [Neurosci. Lett. (2013) 104-109]. Neuroscience Letters, 562, 107.


- Axon guidance effects of classical morphogens Shh and BMP7 in the hypothalamo-pituitary system (DOI:10.1016/j.neulet.2013.08.027). Neuroscience Letters.


- Wild-type but not mutant SOD1 transgenic astrocytes promote the efficient generation of motor neuron progenitors from mouse embryonic stem cells.. BMC Neurosci, 14, 126.


- Axon guidance effect of classical morphogens Shh and BMP7 in the hypothalamo-pituitary system.. Neurosci Lett, 553, 104-109.


- α-Tanycytes of the adult hypothalamic third ventricle include distinct populations of FGF-responsive neural progenitors.. Nat Commun, 4, 2049.


- Direct and indirect roles of Fgf3 and Fgf10 in innervation and vascularisation of the vertebrate hypothalamic neurohypophysis.. Development, 140(5), 1111-1122.


- Development of the medial hypothalamus: forming a functional hypothalamic-neurohypophyseal interface.. Curr Top Dev Biol, 106, 49-88.


- Larysa Pevny, Ph.D., 1965-2012 Obituary. CELL STEM CELL, 12(1), 10-11.


- FGF-dependent midline-derived progenitor cells in hypothalamic infundibular development.. Development, 138(12), 2613-2624.


- FatJ acts via the Hippo mediator Yap1 to restrict the size of neural progenitor cell pools.. Development, 138(10), 1893-1902.


- Molecular pathways controlling development of thalamus and hypothalamus: from neural specification to circuit formation.. J Neurosci, 30(45), 14925-14930.


- Tissue recombinations in collagen gels.. Methods Mol Biol, 461, 325-335.


- Temporal progression of hypothalamic patterning by a dual action of BMP.. Development, 135(20), 3325-3331.


- Regional morphogenesis in the hypothalamus: a BMP-Tbx2 pathway coordinates fate and proliferation through Shh downregulation.. Dev Cell, 11(6), 873-885.


- Orchestrating ontogenesis: variations on a theme by sonic hedgehog. NAT REV GENET, 7(11), 841-850.


- A robust system for RNA interference in the chicken using a modified microRNA operon.. Dev Biol, 294(2), 554-563.


- Directed differentiation of neural cells to hypothalamic dopaminergic neurons.. Development, 132(23), 5185-5197.


- Non-cell-autonomous role for Cripto in axial midline formation during vertebrate embryogenesis.. Development, 132(24), 5539-5551.


- The floor plate: multiple cells, multiple signals.. Nat Rev Neurosci, 6(3), 230-240.


- Molecular control of area a cell specification to anterior floor plate fate. MECHANISMS OF DEVELOPMENT, 122, S164-S165.


- Neurogenesis in the chick hypothalamus. MECHANISMS OF DEVELOPMENT, 122, S177-S177.


- SOX genes and neural progenitor identity.. Curr Opin Neurobiol, 15(1), 7-13.


- Distinct modes of floor plate induction in the chick embryo.. Development, 130(20), 4809-4821.


- Opponent activities of Shh and BMP signaling during floor plate induction in vivo.. Curr Biol, 12(1), 47-52.


- Vertebrate development: Et in Arkadia.. Curr Biol, 11(15), R616-R619.


- Differentiation of ventral midline tissues along the A-P axis. Biochemical Society Transactions, 28(5), A139-A139.


- Development of chick axial mesoderm: specification of prechordal mesoderm by anterior endoderm-derived TGFbeta family signalling.. Development, 127(13), 2795-2809.


- Developmental genetics in Sheffield: a meeting point for Hedgehog researchers.. Int J Dev Biol, 44(1), 65-72.


- Discussion point. The case for floor plate induction by the notochord.. Curr Opin Neurobiol, 10(1), 15-22.


- Rostro-ventral patterning of the neural tube ventral midline. EUROPEAN JOURNAL OF NEUROSCIENCE, 12, 12-12.


- The role of Sonic hedgehog in neural tube patterning.. Cell Mol Life Sci, 57(12), 1695-1708.


- Introduction: preface to the Hedgehog family of proteins review volume. CELL MOL LIFE SCI, 57(12), 1671-1671.


- Airway patterning: A paradigm for restricted signalling.. Curr Biol, 9(14), R506-R510.


- Differential patterning of ventral midline cells by axial mesoderm is regulated by BMP7 and chordin.. Development, 126(2), 397-408.


- Tissue recombinations in collagen gels.. Methods Mol Biol, 97, 293-304.


- Chapter 8 Guidance of developing axons by diffusible chemoattractants. Principles of Medical Biology, 11(C), 153-165.


- The when and where of floor plate induction.. Science, 282(5394), 1654-1657.


- A role for SOX1 in neural determination.. Development, 125(10), 1967-1978.


- Cooperation of BMP7 and SHH in the induction of forebrain ventral midline cells by prechordal mesoderm.. Cell, 90(2), 257-269.


- Patterning cascades in the neural tube. Neural development.. Curr Biol, 6(5), 526-529.


- Sonic hedgehog induces the differentiation of ventral forebrain neurons: a common signal for ventral patterning within the neural tube.. Cell, 81(5), 747-756.


- SONIC HEDGEHOG INDUCES THE DIFFERENTIATION OF VENTRAL FOREBRAIN NEURONS - A COMMON SIGNAL FOR VENTRAL PATTERNING WITHIN THE NEURAL-TUBE (VOL 81, PG 747, 1995). CELL, 82(1), U11-U11.


- Early stages of notochord and floor plate development in the chick embryo defined by normal and induced expression of HNF-3 beta.. Dev Biol, 170(2), 299-313.


- The role of the notochord and floor plate in inductive interactions.. Curr Opin Genet Dev, 5(4), 499-506.


- Floor plate and motor neuron induction by vhh-1, a vertebrate homolog of hedgehog expressed by the notochord.. Cell, 76(4), 761-775.


- Induction of floor plate differentiation by contact-dependent, homeogenetic signals.. Development, 117(1), 205-218.


- Border disputes: do boundaries play a role in growth-cone guidance?. Trends Neurosci, 16(8), 316-323.


- Control of dorsoventral pattern in vertebrate neural development: induction and polarizing properties of the floor plate.. Dev Suppl, Suppl 2, 105-122.


- Control of cell pattern in the developing nervous system: polarizing activity of the floor plate and notochord.. Cell, 64(3), 635-647.


- Target attraction: are developing axons guided by chemotropism?. Trends Neurosci, 14(7), 303-310.


- Mesodermal control of neural cell identity: floor plate induction by the notochord.. Science, 250(4983), 985-988.


- Orientation of commissural axons in vitro in response to a floor plate-derived chemoattractant.. Development, 110(1), 19-30.


- Guidance of developing axons by diffusible chemoattractants.. Cold Spring Harb Symp Quant Biol, 55, 279-289.


- Polarity and patterning in the neural tube: the origin and function of the floor plate.. Ciba Found Symp, 144, 255-276.


- The mouse homolog of the hst/k-FGF gene is adjacent to int-2 and is activated by proviral insertion in some virally induced mammary tumors.. Proc Natl Acad Sci U S A, 86(15), 5678-5682.


- A putative int domain for mouse mammary tumor virus on mouse chromosome 7 is a 5' extension of int-2.. J Virol, 63(3), 1448-1450.


- Chemotropic guidance of developing axons in the mammalian central nervous system.. Nature, 336(6201), 775-778.


- Insertion elements and transitions in cloned mouse mammary tumour virus DNA: further delineation of the poison sequences.. Nucleic Acids Res, 14(21), 8231-8245.


- Characterization, chromosome assignment, and segregation analysis of endogenous proviral units of mouse mammary tumor virus.. J Virol, 59(3), 535-544.


- Resolving forebrain developmental organisation by analysis of differential growth patterns. Nature Communications.


Book chapters
- Organizing activities of axial mesoderm, Current Topics in Developmental Biology (pp. 83-123). Elsevier


- Development of the Neuroendocrine Hypothalamus, Masterclass in Neuroendocrinology (pp. 3-30). Springer International Publishing


- Content Experts, Larsen's Human Embryology (pp. v-v). Elsevier


- Early Development of the Neural Tube, Imaging the Central Nervous System of the Fetus and Neonate (pp. 3-12). CRC Press


- Axonal Pathfinding in the Developing Spinal Cord: Involvement of the Floor Plate in Chemotropic and Contact Guidance, Brain Repair (pp. 199-211). Macmillan Education UK


Conference proceedings
- 1300: Adult neurogenesis is impaired in pink1-/- zebrafish (Danio rerio). Movement Disorders, Vol. 33(S2) (pp S605-S605). Hong Kong, China View this article in WRRO


- Adult stem cells in the human and mouse spinal cord: What are their characteristics and can they migrate and replicate upon exposure to growth factors. BRITISH JOURNAL OF SURGERY, Vol. 100 (pp 38-39)


- FLOW INDUCED VESSEL REMODELLING IN THE CHICKEN EMBRYO IS ASSOCIATED WITH A SPECIFIC GENE EXPRESSION PROFILE. HEART, Vol. 98 (pp A4-A4)


- TRANSCRIPTIONAL PROFILING REVEALS A REQUIREMENT FOR PHOSPHODIESTERASE 10A DURING FLOW-INDUCED VESSEL REMODELLING IN THE CHICK EMBRYO. HEART, Vol. 97(20) (pp 15-15)


- A NOVEL CHICK EMBRYO MODEL REVEALS THAT ENDOTHELIN RECEPTOR B IS ESSENTIAL FOR COLLATERAL VESSEL DEVELOPMENT. HEART, Vol. 95(22)


- Stem cells in the adult hypothalamus. MECHANISMS OF DEVELOPMENT, Vol. 126 (pp S8-S8)


- A temporal integration of BMP signalling controls progressive progenitor specification in the ventral diencephalon. MECHANISMS OF DEVELOPMENT, Vol. 126 (pp S210-S210)


- Regulation of neural stem cells in the zebrafish hypothalamus by Shh. MECHANISMS OF DEVELOPMENT, Vol. 126 (pp S220-S220)


- Small molecule screening of zebrafish models of disease and development. MECHANISMS OF DEVELOPMENT, Vol. 126 (pp S142-S142)


- Development of the ventral hypothalamus and infundibulum. DEVELOPMENTAL BIOLOGY, Vol. 331(2) (pp 467-468)


- A NOVEL CHICK EMBRYO MODEL REVEALS ENDOTHELIN RECEPTOR B IS ESSENTIAL FOR COLLATERAL VESSEL DEVELOPMENT. HEART, Vol. 95 (pp A65-A65)


- A role for BMPs in the formation of the ventral hypothalamus.. DEVELOPMENTAL BIOLOGY, Vol. 259(2) (pp 492-492)


Preprints
- Resolving forebrain developmental organisation by analysis of differential growth patterns, Cold Spring Harbor Laboratory.


- A neuroepithelial wave of BMP signalling drives anteroposterior specification of the tuberal hypothalamus, Cold Spring Harbor Laboratory.


- Accurate staging of chick embryonic tissues via deep learning, Cold Spring Harbor Laboratory.


- The neural cell adhesion molecule NrCAM regulates development of hypothalamic tanycytes, Cold Spring Harbor Laboratory.


- Single-cell analysis of early chick hypothalamic development reveals that hypothalamic cells are induced from prethalamic-like progenitors, Cold Spring Harbor Laboratory.


- Defining the signalling determinants of a posterior ventral spinal cord identity in human neuromesodermal progenitor derivatives, Cold Spring Harbor Laboratory.


- Crumbs2 mediates ventricular layer remodelling to form the adult spinal cord central canal, Cold Spring Harbor Laboratory.


- Regulation of neuron-specific gene transcription by stress hormone signalling requires synaptic activity in zebrafish, Cold Spring Harbor Laboratory.


- Single-Cell Analysis of Early Hypothalamic Development Reveals that Hypothalamic Cells are Induced from Prethalamic-Like Progenitors.


- Accurate staging of chick embryonic tissues via deep learning of salient features. Development, 150(22). View this article in WRRO
- Research group
-
Current lab members:
- Dr Sarah Burbridge (Research Assistant)
- Dr Kavitha Chinnaiya (Research Associate)
- Dr Elizabeth Manning (Research Associate)
- Dr Elsie Place (Research Associate)
- Mr Ian Groves (PhD student; joint with Dr Alex Fletcher)
- Ms Bethany James (PhD student; joint with Professor Andrew Furley and Dr Ivana Barbaric)
- Teaching activities
-
I lecture and teach on several undergraduate and MSc courses, including second, third year and masters courses in developmental biology, developmental neurobiology and stem/ regenerative biology. I am module co-ordinator for Developmental Neurobiology (second year module).
My group annually takes several undergraduate and masters students into the lab for their final dissertation/research projects and most years we have summer students working with us.
I am an invited editor of ‘Principles of Development Biology’ (7th ed).
- Professional activities and memberships
-
- Chair: Wellcome Trust Expert Review Group: Cell and Developmental Biology
- Scientific Advisory Board member: GW4 Biomed MRC DTP
Presentations
I am invited to present research talks at international meetings in numerous fields, including Developmental biology, Neuroscience, Neuroendocrinology, Endocrinology
Examination bodies
- External Examiner for Development, Regeneration and Stem Cells, University of Edinburgh (2016-2019)
- External Examiner for Part 2 Zoology, University of Cambridge (2013-2015)
Professional societies
- British Society for Developmental Biology
- Society for Endocrinology
- Society for Neuroscience
- Society for Anatomy
Peer review activity
I am an ad hoc reviewer for many journals (including Cell, Nature Neuroscience, Current Biology, Development, Developmental Cell, PLoS Biology) and for Grant awarding bodies (including Wellcome Trust, BBSRC, MRC, ERC, Hong Kong Research Council, Human Science Frontiers Program; NIHR). I have been involved in many promotion reviews for scientists in my field of research in the UK, USA and elsewhere, act as a Content Expert (eg Larsen’s Human Embryology 4th Ed (Ed: G. Schoenwolf)) and editor (Wolpert’s Principlels of Developmental Biology 7th Ed).