Professor Sherif El-Khamisy
School of Biosciences
Professor of Molecular Medicine
Deputy Director of the Healthy Lifespan Institute
Full contact details
School of Biosciences
Firth Court
Western Bank
Sheffield
S10 2TN
- Profile
-
Career history
- 2015 - present: Director of Research and Innovation
- 2014 - present: Chair of Molecular Medicine, Krebs Institute, University of Sheffield
- 2014 - present: Wellcome Trust Investigator, Krebs Institute, University of Sheffield
- 2014 - present: Lister Research Fellow, Krebs Institute, University of Sheffield
- 2013 - 2015: Wellcome Trust Group Leader, Genome Center, University of Sussex
- 2013 - 2014: Reader, Krebs Institute, University of Sheffield
- 2008 - 2013: Wellcome Trust Fellow, Genome Center, University of Sussex
- 2007 - 2008: MRC Post-doctoral Fellow, Genome Center, University of Sussex, UK
- 2006: Post-doctoral Fellow, Dept. of Genetics, St Jude Children’s Research Hospital, USA
- 2005: Lecturer, Dept. of Biochemistry, Ain Shams University, Egypt
- 2002 - 2005: PhD in Biochemistry, University of Sussex, UK
Video animations summarising recent research discoveries
This video describes how DNA repair guards us against ALS, in particular the most common genetic cause for ALS and frontotemporal dementia caused by expansion in a gene called C9orf72. It summarises the work published in Nature Neuroscience on 17 July 2017.
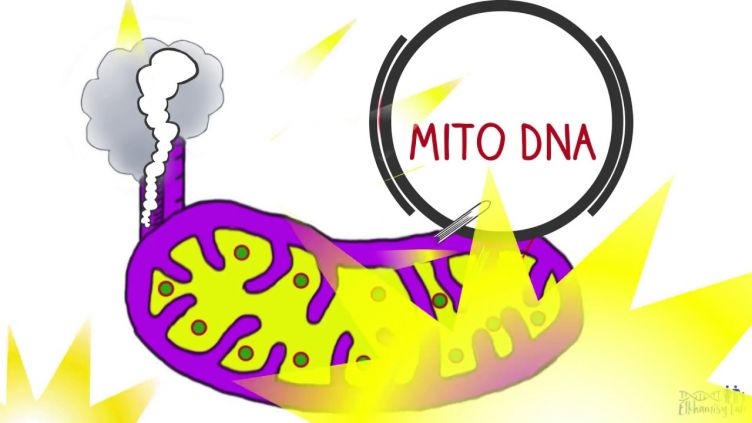
How can the cells' powerhouse - mitochondria - protect their DNA? This video summarises a recent publication in Science Advances.
Major scientific accomplishments
- Discovery of the mechanism of genomic instability and neural cell death in C9orf72 ALS (Nature Neuroscience 2017)
- Discovery of protein-linked chromosomal break repair in the mitochondria (Science Advances 2017)
- Discovery of a novel therapeutic strategy for ALS by inhibiting nuclear export (Nature communications 2017)
- Elucidation of an epigenetic mechanism underlying irinotecan resistance in colorectal cancer (Nucleic Acids Research 2016)
- Development of a nano-genomic technology to diagnose nucleic acid based infections (Biosensors 2016)
- Discovery of the first human diseases resulting from accumulation of Top2-linked DNA breaks (Nature Genetics 2014)
- Elucidation of the mechanisms underlying temozolomide therapy in brain tumors (Nucleic Acids Research 2014)
- Development of techniques to measure protein-linked chromosomal breaks (Plos One 2013)
- Identification of the molecular role of SUMOylation during single-strand break repair (Nature Communications 2012)
- Identification of the role of XRCC1 during neural development and maintenance (Nature Neuroscience 2010)
- Discovery of the enzyme that disjoins abortive topoisomerase 2 DNA breaks (Nature 2009)
- Discovery of the function of the neuroprotective enzyme aprataxin (Nature 2006)
- Identification of the first human disease with defects in chromosomal single-strand break repair (Nature 2005)
- Elucidation of the role of CK2 in chromosomal single-strand break repair (Cell 2003)
- Qualifications
-
Honours and distinctions
- Wellcome Trust Investigator Award
- Fellow of the Royal Society of Chemistry
- Fellow of the Royal Society of Biology
- Fellow of the Lister Institute of Preventative Medicine
- Shoman Award for Medical Sciences
- Sir Richard Stapley Award, UK
- Lorne Duncan Award, UK
- Research interests
-
The intertwined nature of the DNA double helix creates topological barriers that need to be resolved during all types of DNA transactions, in all forms of life from plant to man.
DNA topoisomerases achieve this by breaking and sealing the double helix, allowing the DNA to become untangled or unwound. During their normal catalytic cycle DNA topoisomerases become covalently attached to the DNA 3’-end (Top1) or to the 5’-end (Top2) via a reversible covalent phosphotyrosyl bond.
The presence of nearby oxidative DNA breaks (red circles) or collision with elongating RNA polymerases (RNA POL) during transcription results in ‘trapping’ of topoisomerases on DNA, causing protein-linked DNA breaks (PDBs), which are potent blocks to transcription and cell viability.
PDBs are repaired by a nucleolytic cleavage of DNA releasing the stalled Top and a fragment of DNA. Since this process results in an inevitable loss of genetic material, it is inherently error-prone.
Alternatively, PDBs can be repaired by an error-free mechanism in which the covalent phosphotyrosyl bond linking DNA to the stalled Top is cleaved by specific enzymes, such as tyrosyl DNA phosphodiesterases (TDP1 and TDP2). Interestingly, defects in TDPs cause neurological disease in man (see Fig.2).
In addition to maintaining genetic integrity in post-mitotic non cycling cells, accumulation of PDBs in cycling cells has been widely exploited to treat cancer (e.g. topoisomerase poisons such as Irinotecan and Topotecan), and therefore small molecule inhibitors of TDPs are emerging as attractive strategies to improve cancer therapy.
- Publications
-
Show: Featured publications All publications
Featured publications
Journal articles
- SMN-deficient cells exhibit increased ribosomal DNA damage. Life Science Alliance, 5(8). View this article in WRRO


- Defective repair of topoisomerase I induced chromosomal damage in Huntington’s disease. Cellular and Molecular Life Sciences, 79(3).


- A role for Rad5 in ribonucleoside monophosphate (rNMP) tolerance. Life Science Alliance, 4(10). View this article in WRRO


- USP11 controls R-loops by regulating senataxin proteostasis. Nature Communications, 12.


- Hypoxia-induced SETX links replication stress with the unfolded protein response. Nature Communications, 12(1).


- High temperature drives topoisomerase mediated chromosomal break repair pathway choice. Cancers, 13(10).


- Tdp1 protects from topoisomerase 1–mediated chromosomal breaks in adult zebrafish but is dispensable during larval development. Science Advances, 7(5). View this article in WRRO


- Investigation of the role of VHL-HIF signaling in DNA repair and apoptosis in zebrafish. Oncotarget, 11(13), 1109-1130. View this article in WRRO


- TEX264 coordinates p97- and SPRTN-mediated resolution of topoisomerase 1-DNA adducts. Nature Communications, 11(1), ---.


- Nucleic acids biosensors based on metal-organic framework (MOF): Paving the way to clinical laboratory diagnosis. Biosensors and Bioelectronics, 141, 111451-111451.


- Increased genomic instability following treatment with direct acting anti-hepatitis C virus drugs. EBioMedicine, 35, 106-113. View this article in WRRO


- Rad5, HLTF, and SHPRH: A fresh view of an old story. Trends in Genetics, 34(8), 574-577.


- UVA-induced carbon-centred radicals in lightly pigmented cells detected using ESR spectroscopy. Free Radical Biology and Medicine, 126, 153-165.


- miR-363 confers taxane resistance in ovarian cancer by targeting the Hippo pathway member, LATS2. Oncotarget, 9(53), 30053-30065. View this article in WRRO


- UCHL3 Regulates Topoisomerase-Induced Chromosomal Break Repair by Controlling TDP1 Proteostasis. Cell Reports, 23(11), 3352-3365. View this article in WRRO


- Competing endogenous RNA network crosstalk reveals novel molecular markers in colorectal cancer. Journal of Cellular Biochemistry, 119(8), 6869-6881.


- Perturbed autophagy and DNA repair converge to promote neurodegeneration in amyotrophic lateral sclerosis and dementia. Brain, 141(5), 1247-1262. View this article in WRRO


- Choose your yeast strain carefully: the RAD5 gene matters. Nature Reviews Molecular Cell Biology, 19(6), 343-344.


- C9orf72 Expansion Disrupts ATM-mediated Chromosomal Break Repair. Nature Neuroscience, 20(9), 1225-1235. View this article in WRRO


- SRSF1-dependent nuclear export inhibition of C9ORF72 repeat transcripts prevents neurodegeneration and associated motor deficits. Nature Communications, 8. View this article in WRRO


- Mitochondrial protein-linked DNA breaks perturb mitochondrial gene transcription and trigger free radical-induced DNA damage. Science Advances, 3(4). View this article in WRRO


- Epigenetic changes in histone acetylation underpin resistance to the topoisomerase I inhibitor irinotecan.. Nucleic Acids Research, 45(3), 1159-1176.


- Gold aggregating gold: A novel nanoparticle biosensor approach for the direct quantification of hepatitis C virus RNA in clinical samples. Biosensors and Bioelectronics.


- Chemical screening identifies the β-Carboline Alkaloid Harmine to be synergistically lethal with Doxorubicin. Mechanisms of Ageing and Development.


- Isoeugenol is a selective potentiator of camptothecin cytotoxicity in vertebrate cells lacking TDP1. Scientific Reports, 6. View this article in WRRO


- A Comprehensive Analysis of the Dynamic Response to Aphidicolin-Mediated Replication Stress Uncovers Targets for ATM and ATMIN. Cell Reports, 15(4), 893-908. View this article in WRRO


- Topoisomerase-mediated chromosomal break repair: an emerging player in many games. Nature Reviews Cancer, 15(3), 137-151.


- Clinical and Cellular Roles for TDP1 and TOP1 in Modulating Colorectal Cancer Response to Irinotecan. Molecular Cancer Therapeutics, 14(2), 575-585.


- Expression of a pathogenic mutation of SOD1 sensitizes aprataxin-deficient cells and mice to oxidative stress and triggers hallmarks of premature ageing. Human Molecular Genetics, 24(3), 828-840. View this article in WRRO


- TDP2 protects transcription from abortive topoisomerase activity and is required for normal neural function. Nature Genetics, 46(5), 516-521.


- TDP1 deficiency sensitizes human cells to base damage via distinct topoisomerase I and PARP mechanisms with potential applications for cancer therapy. Nucleic Acids Research, 42(5), 3089-3103. View this article in WRRO


- ATM deficiency results in accumulation of DNA-topoisomerase I covalent intermediates in neural cells.. PLoS One, 8(4), e58239.


- TDP2 promotes repair of topoisomerase I-mediated DNA damage in the absence of TDP1.. Nucleic Acids Res, 40(17), 8371-8380.


- SUMO modification of the neuroprotective protein TDP1 facilitates chromosomal single-strand break repair.. Nat Commun, 3, 733.


- To live or to die: a matter of processing damaged DNA termini in neurons.. EMBO Mol Med, 3(2), 78-88.


- TDP2/TTRAP is the major 5'-tyrosyl DNA phosphodiesterase activity in vertebrate cells and is critical for cellular resistance to topoisomerase II-induced DNA damage.. J Biol Chem, 286(1), 403-409.


- Mutations in Cullin 4B result in a human syndrome associated with increased camptothecin-induced topoisomerase I-dependent DNA breaks.. Hum Mol Genet, 19(7), 1324-1334.


- A human 5'-tyrosyl DNA phosphodiesterase that repairs topoisomerase-mediated DNA damage.. Nature, 461(7264), 674-678.


- The genesis of cerebellar interneurons and the prevention of neural DNA damage require XRCC1.. Nat Neurosci, 12(8), 973-980.


- TDP1 facilitates chromosomal single-strand break repair in neurons and is neuroprotective in vivo.. EMBO J, 26(22), 4720-4731.


- The neurodegenerative disease protein aprataxin resolves abortive DNA ligation intermediates.. Nature, 443(7112), 713-716.


- Defective DNA single-strand break repair in spinocerebellar ataxia with axonal neuropathy-1. NATURE, 434(7029), 108-113.


- The protein kinase CK2 facilitates repair of chromosomal DNA single-strand breaks.. Cell, 117(1), 17-28.


- A requirement for PARP-1 for the assembly or stability of XRCC1 nuclear foci at sites of oxidative DNA damage.. Nucleic Acids Res, 31(19), 5526-5533.


All publications
Journal articles
- Decoding the adaptive survival mechanisms of breast cancer dormancy. Oncogene, 44, 3759-3773. View this article in WRRO


- Senataxin regulates cisplatin resistance through an R-loop-mediated mechanism in HPV-associated head and neck cancer. iScience, 28(9). View this article in WRRO


- ADP-ribosylation of NuMA promotes DNA single-strand break repair and transcription. Cell Reports, 44(6), 115737-115737.


- Insights into the human cDNA: A descriptive study using library screening in yeast. Journal of Genetic Engineering and Biotechnology, 22(4), 100427-100427.


- 540P Assessing the effect of chronic nitrate supplementation on muscle mass and physical function outcomes. Neuromuscular Disorders, 43, 104441.131-104441.131.


- Second generation lethality in RNAseH2a knockout zebrafish. Nucleic Acids Research, 52(18), 11014-11028. View this article in WRRO


- Genomic landscape of hepatocellular carcinoma in Egyptian patients by whole exome sequencing. BMC Medical Genomics, 17. View this article in WRRO


- Hypoxia‐responsive prodrug of ATR inhibitor, AZD6738, selectively eradicates treatment‐resistant cancer cells. Advanced Science, 11(34). View this article in WRRO


- The DNA repair kinase ATM regulates CD13 expression and cell migration. Frontiers in Cell and Developmental Biology, 12. View this article in WRRO


- A comparative study of different antiviral treatment protocols in HCV related cryoglobulinemic vasculitis. Scientific Reports, 14(1). View this article in WRRO


- Chromosomal single-strand break repair and neurological disease: Implications on transcription and emerging genomic tools. DNA Repair, 135. View this article in WRRO


- Abstract A023: High glucose increases DNA damage and elevates the expression of multiple DDR genes. Cancer Research, 84(1_Supplement), A023-A023.


- Cancer-specific glycosylation of CD13 impacts its detection and activity in preclinical cancer tissues. iScience, 26(11). View this article in WRRO


- Exercise Interventions for the Management of Sarcopenia: Possibilities and Challenges. Physical & Occupational Therapy In Geriatrics, 41(4), 654-677.


- Nutritional Supplementation for the Prevention of Muscle Atrophy in Older People. Nutrition Today, 58(3), 105-118.


- Oxidative DNA damage and repair at non-coding regulatory regions. Trends in Cell Biology, 33(11), 939-949. View this article in WRRO


- High glucose increases DNA damage and elevates the expression of multiple DDR genes. Genes, 14(1).


- Genome-wide association study for systemic lupus erythematosus in an egyptian population. Frontiers in Genetics, 13.


- A mechanism for oxidative damage repair at gene regulatory elements. Nature, 609(7929), 1038-1047.


- Nanomedicines targeting the inflammasome as a promising therapeutic approach for cell senescence. Seminars in Cancer Biology, 86, 46-53.


- CORM-3 induces DNA damage through Ru(II) binding to DNA. Biochemical Journal, 479(13), 1429-1439. View this article in WRRO


- Abstract 1049: Modulating the polymorphic UGT1A1 gene to enhance camptothecin anti-colorectal effect. Cancer Research, 82(12_Supplement), 1049-1049.


- Editorial: Genomic instability and neurodegeneration. Frontiers in Aging Neuroscience, 14.


- SMN-deficient cells exhibit increased ribosomal DNA damage. Life Science Alliance, 5(8). View this article in WRRO


- Defective repair of topoisomerase I induced chromosomal damage in Huntington’s disease. Cellular and Molecular Life Sciences, 79(3).


- Gene of the month: NKX3.1. Journal of Clinical Pathology, 75(6), 361-364. View this article in WRRO


- Impact of circ-0000221 in the pathogenesis of hepatocellular via modulation of miR-661–PTPN11 mRNA axis. Pharmaceutics, 14(1).


- A role for Rad5 in ribonucleoside monophosphate (rNMP) tolerance. Life Science Alliance, 4(10). View this article in WRRO


- USP11 controls R-loops by regulating senataxin proteostasis. Nature Communications, 12.


- DNA damage as a mechanism of neurodegeneration in ALS and a contributor to astrocyte toxicity. Cellular and Molecular Life Sciences, 78(15), 5707-5729. View this article in WRRO


- Apurinic/Apyrimidinic Endonuclease 2 (APE2): An ancillary enzyme for contextual base excision repair mechanisms to preserve genome stability. Biochimie, 190, 70-90.


- Hypoxia-induced SETX links replication stress with the unfolded protein response. Nature Communications, 12(1).


- High temperature drives topoisomerase mediated chromosomal break repair pathway choice. Cancers, 13(10).


- Identification and validation of ERK5 as a DNA damage modulating drug target in glioblastoma. Cancers, 13(5). View this article in WRRO


- Tdp1 protects from topoisomerase 1–mediated chromosomal breaks in adult zebrafish but is dispensable during larval development. Science Advances, 7(5). View this article in WRRO


- Integrative microRNA and gene expression analysis identifies new epigenetically regulated microRNAs mediating taxane resistance in ovarian cancer. Scientific Reports, 11(1).


- Nucleosides rescue replication-mediated genome instability of human pluripotent stem cells. Stem Cell Reports, 14(6), 1009-1017. View this article in WRRO


- Investigation of the role of VHL-HIF signaling in DNA repair and apoptosis in zebrafish. Oncotarget, 11(13), 1109-1130. View this article in WRRO


- TEX264 coordinates p97- and SPRTN-mediated resolution of topoisomerase 1-DNA adducts. Nature Communications, 11(1), ---.


- Typhoid toxin exhausts the RPA response to DNA replication stress driving senescence and Salmonella infection. Nature Communications, 10(1). View this article in WRRO


- DNA repair and neurological disease: From molecular understanding to the development of diagnostics and model organisms. DNA Repair, 81, 102669-102669.


- Nucleic acids biosensors based on metal-organic framework (MOF): Paving the way to clinical laboratory diagnosis. Biosensors and Bioelectronics, 141, 111451-111451.


- A new filter-based Gene selection method based on dragonfly optimization and correlation-based feature selection. BIOSCIENCE RESEARCH, 16(3), 3139-3154.


- RETRACTED: Study of the mechanisms of crocetin‐induced differentiation and apoptosis in human acute promyelocytic leukemia cells. Journal of Cellular Biochemistry, 120(2), 1943-1957.


- Recent advances in stem cells therapy: A focus on cancer, Parkinson’s and Alzheimer’s. Journal of Genetic Engineering and Biotechnology, 16(2), 427-432.


- Gold nanoparticles - an optical biosensor for RNA quantification for cancer and neurologic disorders diagnosis. International Journal of Nanomedicine, 13, 8137-8151.


- Increased genomic instability following treatment with direct acting anti-hepatitis C virus drugs. EBioMedicine, 35, 106-113. View this article in WRRO


- Rad5, HLTF, and SHPRH: A fresh view of an old story. Trends in Genetics, 34(8), 574-577.


- UVA-induced carbon-centred radicals in lightly pigmented cells detected using ESR spectroscopy. Free Radical Biology and Medicine, 126, 153-165.


- miR-363 confers taxane resistance in ovarian cancer by targeting the Hippo pathway member, LATS2. Oncotarget, 9(53), 30053-30065. View this article in WRRO


- UCHL3 Regulates Topoisomerase-Induced Chromosomal Break Repair by Controlling TDP1 Proteostasis. Cell Reports, 23(11), 3352-3365. View this article in WRRO


- Competing endogenous RNA network crosstalk reveals novel molecular markers in colorectal cancer. Journal of Cellular Biochemistry, 119(8), 6869-6881.


- Perturbed autophagy and DNA repair converge to promote neurodegeneration in amyotrophic lateral sclerosis and dementia. Brain, 141(5), 1247-1262. View this article in WRRO


- Choose your yeast strain carefully: the RAD5 gene matters. Nature Reviews Molecular Cell Biology, 19(6), 343-344.


- Studying TDP1 Function in DNA Repair, 173-181.


- Building Bridges through Science. Neuron, 96(4), 730-735.


- C9orf72 Expansion Disrupts ATM-mediated Chromosomal Break Repair. Nature Neuroscience, 20(9), 1225-1235. View this article in WRRO


- Role of Protein Linked DNA Breaks in Cancer, 41-58.


- Viral delivery of C9ORF72 hexanucleotide repeat expansions in mice lead to repeat length dependent neuropathology and behavioral deficits.. Disease Models and Mechanisms. View this article in WRRO


- SRSF1-dependent nuclear export inhibition of C9ORF72 repeat transcripts prevents neurodegeneration and associated motor deficits. Nature Communications, 8. View this article in WRRO


- Mitochondrial protein-linked DNA breaks perturb mitochondrial gene transcription and trigger free radical-induced DNA damage. Science Advances, 3(4). View this article in WRRO


- Epigenetic changes in histone acetylation underpin resistance to the topoisomerase I inhibitor irinotecan.. Nucleic Acids Research, 45(3), 1159-1176.


- Gold aggregating gold: A novel nanoparticle biosensor approach for the direct quantification of hepatitis C virus RNA in clinical samples. Biosensors and Bioelectronics.


- Chemical screening identifies the β-Carboline Alkaloid Harmine to be synergistically lethal with Doxorubicin. Mechanisms of Ageing and Development.


- Isoeugenol is a selective potentiator of camptothecin cytotoxicity in vertebrate cells lacking TDP1. Scientific Reports, 6. View this article in WRRO


- A Comprehensive Analysis of the Dynamic Response to Aphidicolin-Mediated Replication Stress Uncovers Targets for ATM and ATMIN. Cell Reports, 15(4), 893-908. View this article in WRRO


- P18/Stathmin1 is regulated by miR-31 in ovarian cancer in response to taxane.. Oncoscience, 2(3), 294-308. View this article in WRRO


- Topoisomerase-mediated chromosomal break repair: an emerging player in many games. Nature Reviews Cancer, 15(3), 137-151.


- Clinical and Cellular Roles for TDP1 and TOP1 in Modulating Colorectal Cancer Response to Irinotecan. Molecular Cancer Therapeutics, 14(2), 575-585.


- Expression of a pathogenic mutation of SOD1 sensitizes aprataxin-deficient cells and mice to oxidative stress and triggers hallmarks of premature ageing. Human Molecular Genetics, 24(3), 828-840. View this article in WRRO


- DNA Damage Response Pathways in Cancer Predisposition and Progression, 39-74.


- TDP1/TOP1 Ratio as a Promising Indicator for the Response of Small Cell Lung Cancer to Topotecan.. Journal of Cancer Science and Therapy, 6(7), 258-267. View this article in WRRO


- TDP2 protects transcription from abortive topoisomerase activity and is required for normal neural function. Nature Genetics, 46(5), 516-521.


- Development of an oligonucleotide-based fluorescence assay for the identification of tyrosyl-DNA phosphodiesterase 1 (TDP1) inhibitors. Analytical Biochemistry, 454, 17-22.


- TDP1 deficiency sensitizes human cells to base damage via distinct topoisomerase I and PARP mechanisms with potential applications for cancer therapy. Nucleic Acids Research, 42(5), 3089-3103. View this article in WRRO


- ATM deficiency results in accumulation of DNA-topoisomerase I covalent intermediates in neural cells.. PLoS One, 8(4), e58239.


- TDP2 promotes repair of topoisomerase I-mediated DNA damage in the absence of TDP1.. Nucleic Acids Res, 40(17), 8371-8380.


- DNA repair and resistance to topoisomerase I inhibitors: mechanisms, biomarkers and therapeutic targets.. Curr Med Chem, 19(23), 3874-3885.


- Tyrosyl-DNA phosphodiesterase 1 initiates repair of apurinic/apyrimidinic sites.. Biochimie, 94(8), 1749-1753.


- SUMO modification of the neuroprotective protein TDP1 facilitates chromosomal single-strand break repair.. Nat Commun, 3, 733.


- Topoisomerase I inhibition in colorectal cancer: biomarkers and therapeutic targets.. Br J Cancer, 106(1), 18-24.


- To live or to die: a matter of processing damaged DNA termini in neurons.. EMBO Mol Med, 3(2), 78-88.


- TDP2/TTRAP is the major 5'-tyrosyl DNA phosphodiesterase activity in vertebrate cells and is critical for cellular resistance to topoisomerase II-induced DNA damage.. J Biol Chem, 286(1), 403-409.


- TDP1 serine 81 promotes interaction with DNA ligase IIIalpha and facilitates cell survival following DNA damage.. Cell Cycle, 9(3), 588-595.


- Mutations in Cullin 4B result in a human syndrome associated with increased camptothecin-induced topoisomerase I-dependent DNA breaks.. Hum Mol Genet, 19(7), 1324-1334.


- Defective DNA ligation during short-patch single-strand break repair in ataxia oculomotor apraxia 1.. Mol Cell Biol, 29(5), 1354-1362.


- A human 5'-tyrosyl DNA phosphodiesterase that repairs topoisomerase-mediated DNA damage.. Nature, 461(7264), 674-678.


- The genesis of cerebellar interneurons and the prevention of neural DNA damage require XRCC1.. Nat Neurosci, 12(8), 973-980.


- Short-patch single-strand break repair in ataxia oculomotor apraxia-1.. Biochem Soc Trans, 37(Pt 3), 577-581.


- Synergistic decrease of DNA single-strand break repair rates in mouse neural cells lacking both Tdp1 and aprataxin.. DNA Repair (Amst), 8(6), 760-766.


- REPAIR OF DNA SINGLE-STRAND BREAKS IN NEURONS: IMPLICATIONS ON HUMAN HEALTH. CELLULAR ONCOLOGY, 31(2), 131-131.


- APLF (C2orf13) is a novel human protein involved in the cellular response to chromosomal DNA strand breaks (Molecular and Cellular Biology (2007) 27, 10, (3793-3803)). Molecular and Cellular Biology, 28(10), 3561.


- APLF (C2orf13) is a novel human protein involved in the cellular response to chromosomal DNA strand breaks.. Mol Cell Biol, 27(10), 3793-3803.


- TDP1 facilitates chromosomal single-strand break repair in neurons and is neuroprotective in vivo.. EMBO J, 26(22), 4720-4731.


- TDP1 facilitates repair of ionizing radiation-induced DNA single-strand breaks.. DNA Repair (Amst), 6(10), 1485-1495.


- Characterisation of aprataxin- and PNK-like factor: a novel FHA-domain protein involved in single and double-strand break repair. MUTAGENESIS, 22(6), 449-450.


- DNA single-strand break repair and spinocerebellar ataxia with axonal neuropathy-1.. Neuroscience, 145(4), 1260-1266.


- The neurodegenerative disease protein aprataxin resolves abortive DNA ligation intermediates.. Nature, 443(7112), 713-716.


- Measurement of chromosomal DNA single-strand breaks and replication fork progression rates.. Methods Enzymol, 409, 410-425.


- TDP1-dependent DNA single-strand break repair and neurodegeneration.. Mutagenesis, 21(4), 219-224.


- Defective DNA single-strand break repair in spinocerebellar ataxia with axonal neuropathy-1. NATURE, 434(7029), 108-113.


- The protein kinase CK2 facilitates repair of chromosomal DNA single-strand breaks.. Cell, 117(1), 17-28.


- A requirement for PARP-1 for the assembly or stability of XRCC1 nuclear foci at sites of oxidative DNA damage.. Nucleic Acids Res, 31(19), 5526-5533.


- DNA Homeostasis and Senescence: Lessons from the Naked Mole Rat. International Journal of Molecular Sciences, 22(11), 6011-6011.


- A thiol-reactive Ru(II) ion, not CO release, underlies the potent antimicrobial and cytotoxic properties of CO-releasing molecule-3. Redox Biology, 18, 114-123. View this article in WRRO


- https://www.omicsonline.org/open-access/biodegradable-nanocapsules-containing-a-nanobiotechnological-complex-for-the-invitro-suppression-of-a-melanoma-cell-line-b16f10-JNCR-1000102.php?aid=76290. Journal of Nanosciences: Current Research, 01(01).


Book chapters
- Animal Models in Monoclonal Immunoglobulin-Related Diseases, Paraproteinemia and Related Disorders (pp. 57-77). Springer International Publishing


- Gene Therapy in the Nervous System: Failures and Successes In El-Khamisy S (Ed.), Personalised Medicine Lessons from Neurodegeneration to Cancer Springer


- Can astrocytes be a target for precision medicine? In El-Khamisy S (Ed.), Personalised Medicine Springer View this article in WRRO


- Personalised Medicine Lessons from Neurodegeneration to Cancer Preface, PERSONALISED MEDICINE: LESSONS FROM NEURODEGENERATION TO CANCER (pp. V-V).


- Personalised Medicine: Genome Maintenance Lessons Learned from Studies in Yeast as a Model Organism, Advances in Experimental Medicine and Biology (pp. 157-178). Springer International Publishing


- Autosomal Recessive Ataxias Due to Defects in DNA Repair, Movement Disorders (pp. 1033-1041). Elsevier


- Autosomal Recessive Ataxias Due to Defects in DNA Repair, Movement Disorders Genetics and Models Second Edition (pp. 1033-1041).


Conference proceedings
- Immunohistochemical analysis of R-loop burden in a cohort of Oropharyngeal Squamous Cell Carcinomas (OPSCC) with a poor prognosis. VIRCHOWS ARCHIV, Vol. 485 (pp S404-S405)


- The Role of R-loops in the Development of Cisplatin Resistance in Oropharyngeal Squamous Cell Carcinoma (OPSCC). JOURNAL OF PATHOLOGY, Vol. 261(SUPPL1) (pp S62-S62)


- Exploration of the transcriptomic landscape of HPV-positive and HPV-negative oropharyngeal squamous cell carcinoma upon development of cisplatin resistance. VIRCHOWS ARCHIV, Vol. 481(SUPPL 1) (pp S73-S73)


- Modulating the polymorphic UGT1A1 gene to enhance camptothecin anti-colorectal effect. CANCER RESEARCH, Vol. 82(12)


- CISPLATIN RESISTANCE IN HPV-POSITIVE AND HPV-NEGATIVE OROPHARYNGEAL SQUAMOUS CELL CARCINOMA. Oral Surgery, Oral Medicine, Oral Pathology and Oral Radiology, Vol. 132(1) (pp e15-e15)


- Abstract 2512: BRCA1 and BRCA2 mutations and its clinical relevance among egyptian breast cancer women. Cancer Research, Vol. 81(13_Supplement) (pp 2512-2512)


- BRCA1 and BRCA2 mutations and its clinical relevance among egyptian breast cancer women.. CANCER RESEARCH, Vol. 81(13)


- The effect of E6 and E7 knockdown and PARP inhibition on cisplatin sensitivity in oropharyngeal squamous cell carcinoma. VIRCHOWS ARCHIV, Vol. 477 (pp S76-S76)


- Al-Metyaf. Proceedings of the 17th International Conference on Mobile and Ubiquitous Multimedia (pp 391-396)


- DNA Damage and Repair in Patients with Cryoglobulinemic Vasculitis Treated with Direct Anti-HCV Drugs. ARTHRITIS & RHEUMATOLOGY, Vol. 70


- Abstract LB-323: Comparative miRNA microarray profiling indicates miR-363 promotes chemoresistance in ovarian cancer cells by targeting the Hippo member, LATS2. Cancer Research, Vol. 77(13_Supplement)


- COMPARATIVE MIRNA MICROARRAY PROFILING INDICATES MIR-363 PROMOTES CHEMORESISTANCE IN OVARIAN CANCER CELLS BY TARGETING THE HIPPO MEMBER, LATS2. INTERNATIONAL JOURNAL OF GYNECOLOGICAL CANCER, Vol. 27 (pp 1952-1952)


- Treatment of Cryoglobulinemic Vasculitis with Sofosbuvir in Four Combination Protocols. ARTHRITIS & RHEUMATOLOGY, Vol. 68


- Tyrosyl-DNA phosphodiesterase 1-a new player in base excision repair. FEBS JOURNAL, Vol. 280 (pp 63-64)


Preprints
- Senataxin modulates resistance to cisplatin through an R-loop mediated mechanism in HPV-associated Head and Neck Squamous Cell Carcinoma, Cold Spring Harbor Laboratory.


- Exercise Interventions for the Management of Sarcopenia: Possibilities and Challenges, Springer Science and Business Media LLC.


- The investigation of the role of VHL-HIF signaling in DNA repair and apoptosis in zebrafish, Cold Spring Harbor Laboratory.


- Competing endogenous RNA network: Potential entrants to gene editing in Hepatocellular Carcinoma Gene editing of ceRNA in Hepatocellular Carcinoma, Cold Spring Harbor Laboratory.


- SMN-deficient cells exhibit increased ribosomal DNA damage. Life Science Alliance, 5(8). View this article in WRRO
- Research group
-
I welcome applications from self-funded prospective home and international PhD students; see examples of possible projects below.
You can apply for a PhD position in MBB here.
Contact me at S.El-Khamisy@sheffield.ac.uk for further information.
- Teaching activities
-
Level 3 Modules
MBB313 Genome Stability and Genetic Change
Level 2 Modules
MBB262 Genetics 2